Viral-toxin interactions and Parkinson's disease: poly I:C priming enhanced the neurodegenerative effects of paraquat
- PMID: 22559812
- PMCID: PMC3464726
- DOI: 10.1186/1742-2094-9-86
Viral-toxin interactions and Parkinson's disease: poly I:C priming enhanced the neurodegenerative effects of paraquat
Abstract
Background: Parkinson's disease (PD) has been linked with exposure to a variety of environmental and immunological insults (for example, infectious pathogens) in which inflammatory and oxidative processes seem to be involved. In particular, epidemiological studies have found that pesticide exposure and infections may be linked with the incidence of PD. The present study sought to determine whether exposure to a viral mimic prior to exposure to pesticides would exacerbate PD-like pathology.
Methods: Mice received a supra-nigral infusion of 5 μg of the double-stranded RNA viral analog, polyinosinic: polycytidylic acid (poly(I:C)), followed 2, 7 or 14 days later by administration of the pesticide, paraquat (nine 10 mg/kg injections over three weeks).
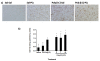
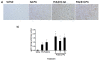
Results: As hypothesized, poly(I:C) pre-treatment enhanced dopamine (DA) neuron loss in the substantia nigra pars compacta elicited by subsequent paraquat treatment. The augmented neuronal loss was accompanied by robust signs of microglial activation, and by increased expression of the catalytic subunit (gp91) of the NADPH oxidase oxidative stress enzyme. However, the paraquat and poly(I:C) treatments did not appreciably affect home-cage activity, striatal DA terminals, or subventricular neurogenesis.
Conclusions: These findings suggest that viral agents can sensitize microglial-dependent inflammatory responses, thereby rendering nigral DA neurons vulnerable to further environmental toxin exposure.
Figures
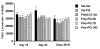

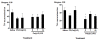
References
-
- Lev N, Melamed E, Offen D. Apoptosis and Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:245–250. - PubMed
-
- McNaught KS, Olanow CW, Halliwell B, Isacson O, Jenner P. Failure of the ubiquitin-proteosome system in Parkinson’s disease. Nat Rev Neurosci. 2001;2:589–594. - PubMed
-
- Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson’s disease. J Neural Transm Suppl. 2000;60:277–290. - PubMed
-
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 1999;22:123–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

