Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation
- PMID: 22560076
- PMCID: PMC3348626
- DOI: 10.1016/j.stem.2012.04.007
Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation
Abstract
The decline in hematopoietic function seen during aging involves a progressive reduction in the immune response and an increased incidence of myeloid malignancy, and has been linked to aging of hematopoietic stem cells (HSCs). The molecular mechanisms underlying HSC aging remain unclear. Here we demonstrate that elevated activity of the small RhoGTPase Cdc42 in aged HSCs is causally linked to HSC aging and correlates with a loss of polarity in aged HSCs. Pharmacological inhibition of Cdc42 activity functionally rejuvenates aged HSCs, increases the percentage of polarized cells in an aged HSC population, and restores the level and spatial distribution of histone H4 lysine 16 acetylation to a status similar to that seen in young HSCs. Our data therefore suggest a mechanistic role for Cdc42 activity in HSC biology and epigenetic regulation, and identify Cdc42 activity as a pharmacological target for ameliorating stem cell aging.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
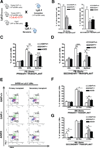
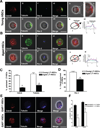



Comment in
-
Restoring cell polarity: an HSC fountain of youth.Cell Stem Cell. 2012 May 4;10(5):481-2. doi: 10.1016/j.stem.2012.04.015. Cell Stem Cell. 2012. PMID: 22560067
References
-
- Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. - PubMed
-
- Alberts AS, Geneste O, Treisman R. Activation of SRF-regulated chromosomal templates by Rho-family GTPases requires a signal that also induces H4 hyperacetylation. Cell. 1998;92:475–487. - PubMed
-
- Barfod ET, Zheng Y, Kuang WJ, Hart MJ, Evans T, Cerione RA, Ashkenazi A. Cloning and expression of a human CDC42 GTPase-activating protein reveals a functional SH3-binding domain. J Biol Chem. 1993;268:26059–26062. - PubMed
-
- Beghe C, Wilson A, Ershler WB. Prevalence and outcomes of anemia in geriatrics: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):3S–10S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

