Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice
- PMID: 22560081
- PMCID: PMC3348507
- DOI: 10.1016/j.stem.2012.02.015
Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice
Abstract
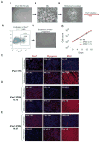
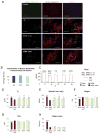
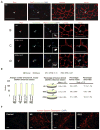
A major obstacle in the application of cell-based therapies for the treatment of neuromuscular disorders is obtaining the appropriate number of stem/progenitor cells to produce effective engraftment. The use of embryonic stem (ES) or induced pluripotent stem (iPS) cells could overcome this hurdle. However, to date, derivation of engraftable skeletal muscle precursors that can restore muscle function from human pluripotent cells has not been achieved. Here we applied conditional expression of PAX7 in human ES/iPS cells to successfully derive large quantities of myogenic precursors, which, upon transplantation into dystrophic muscle, are able to engraft efficiently, producing abundant human-derived DYSTROPHIN-positive myofibers that exhibit superior strength. Importantly, transplanted cells also seed the muscle satellite cell compartment, and engraftment is present over 11 months posttransplant. This study provides the proof of principle for the derivation of functional skeletal myogenic progenitors from human ES/iPS cells and highlights their potential for future therapeutic application in muscular dystrophies.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13:642–648. - PubMed
-
- Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191:270–283. - PubMed
-
- Coulton GR, Rogers B, Strutt P, Skynner MJ, Watt DJ. In situ localisation of single-stranded DNA breaks in nuclei of a subpopulation of cells within regenerating skeletal muscle of the dystrophic mdx mouse. J Cell Sci. 1992;102(Pt 3):653–662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

