Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells
- PMID: 22560445
- PMCID: PMC3716276
- DOI: 10.1016/j.immuni.2012.03.018
Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells
Abstract
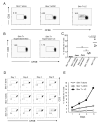
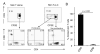
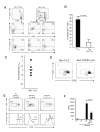
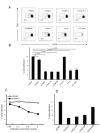
Recent studies have demonstrated that the skin of a normal adult human contains 10-20 billion resident memory T cells, including various helper, cytotoxic, and regulatory T cell subsets, that are poised to respond to environmental antigens. Using only autologous human tissues, we report that both in vitro and in vivo, resting epidermal Langerhan cells (LCs) selectively and specifically induced the activation and proliferation of skin resident regulatory T (Treg) cells, a minor subset of skin resident memory T cells. In the presence of foreign pathogen, however, the same LCs activated and induced proliferation of effector memory T (Tem) cells and limited Treg cells' activation. These underappreciated properties of LCs, namely maintenance of tolerance in normal skin, and activation of protective skin resident memory T cells upon infectious challenge, help clarify the role of LCs in skin.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
-
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. - PubMed
-
- Baecher-Allan CM, Hafler DA. The purification and functional analysis of human CD4+CD25high regulatory T cells. Curr Protoc Immunol. 2006 Chapter 7, Unit 7 4B. - PubMed
-
- Bond E, Adams WC, Smed-Sorensen A, Sandgren KJ, Perbeck L, Hofmann A, Andersson J, Lore K. Techniques for time-efficient isolation of human skin dendritic cell subsets and assessment of their antigen uptake capacity. J Immunol Methods. 2009;348:42–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01AI097218/AI/NIAID NIH HHS/United States
- R01 AI097128/AI/NIAID NIH HHS/United States
- T32 AR007098/AR/NIAMS NIH HHS/United States
- R01 AR065807/AR/NIAMS NIH HHS/United States
- P50 CA093683/CA/NCI NIH HHS/United States
- P30 AR042689/AR/NIAMS NIH HHS/United States
- R03 MH095529/MH/NIMH NIH HHS/United States
- K08 AI060890/AI/NIAID NIH HHS/United States
- R01AI041707/AI/NIAID NIH HHS/United States
- R01 AI041707/AI/NIAID NIH HHS/United States
- R03MH095529/MH/NIMH NIH HHS/United States
- R01 AR056720/AR/NIAMS NIH HHS/United States
- R01 AI025082/AI/NIAID NIH HHS/United States
- L30 AI062111/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

