Myogenesis in dysferlin-deficient myoblasts is inhibited by an intrinsic inflammatory response
- PMID: 22560623
- PMCID: PMC4147957
- DOI: 10.1016/j.nmd.2012.03.002
Myogenesis in dysferlin-deficient myoblasts is inhibited by an intrinsic inflammatory response
Abstract
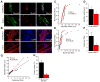
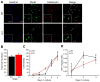
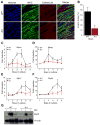
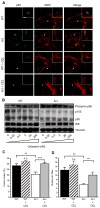
Limb-girdle muscular dystrophy type 2B results from mutations in dysferlin, a membrane-associated protein involved in cellular membrane repair. Primary myoblast cultures derived from dysferlinopathy patients show reduced myogenic potential, suggesting that dysferlin may regulate myotube fusion and be required for muscle regeneration. These observations contrast with the findings that muscle develops normally in pre-symptomatic dysferlinopathy patients. To better understand the role of dysferlin in myogenesis, we investigated this process in vitro using cells derived from two mouse models of dysferlinopathy: SJL/J and A/J mice. We observed that myotubes derived from dysferlin-deficient muscle were of significantly smaller diameters, contained fewer myonuclei, and displayed reduced myogenic gene expression compared to dysferlin-sufficient cells. Together, these findings suggest that the absence of dysferlin from myoblasts is detrimental to myogenesis. Pro-inflammatory NFκB signaling was upregulated in dysferlin-deficient myotubes; the anti-inflammatory agent celastrol reduced the NFκB activation and improved myogenesis in dysferlin-deficient cultures. The results suggest that decreased myotube fusion in dysferlin deficiency is attributable to intrinsic inflammatory activation and can be improved using anti-inflammatory mediators.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Attenuated muscle regeneration is a key factor in dysferlin-deficient muscular dystrophy.Hum Mol Genet. 2009 Jun 1;18(11):1976-89. doi: 10.1093/hmg/ddp121. Epub 2009 Mar 13. Hum Mol Genet. 2009. PMID: 19286669 Free PMC article.
-
Inhibition of inflammation with celastrol fails to improve muscle function in dysferlin-deficient A/J mice.J Neurol Sci. 2015 Sep 15;356(1-2):157-62. doi: 10.1016/j.jns.2015.06.042. Epub 2015 Jun 24. J Neurol Sci. 2015. PMID: 26119397 Free PMC article.
-
Treatment with galectin-1 improves myogenic potential and membrane repair in dysferlin-deficient models.PLoS One. 2020 Sep 3;15(9):e0238441. doi: 10.1371/journal.pone.0238441. eCollection 2020. PLoS One. 2020. PMID: 32881965 Free PMC article.
-
Ferlin proteins in myoblast fusion and muscle growth.Curr Top Dev Biol. 2011;96:203-30. doi: 10.1016/B978-0-12-385940-2.00008-5. Curr Top Dev Biol. 2011. PMID: 21621072 Free PMC article. Review.
-
Dysferlin function in skeletal muscle: Possible pathological mechanisms and therapeutical targets in dysferlinopathies.Exp Neurol. 2016 Sep;283(Pt A):246-54. doi: 10.1016/j.expneurol.2016.06.026. Epub 2016 Jun 25. Exp Neurol. 2016. PMID: 27349407 Review.
Cited by
-
Reduced Sarcolemmal Membrane Repair Exacerbates Striated Muscle Pathology in a Mouse Model of Duchenne Muscular Dystrophy.Cells. 2022 Apr 22;11(9):1417. doi: 10.3390/cells11091417. Cells. 2022. PMID: 35563723 Free PMC article.
-
Serum exosomes can restore cellular function in vitro and be used for diagnosis in dysferlinopathy.Theranostics. 2018 Feb 2;8(5):1243-1255. doi: 10.7150/thno.22856. eCollection 2018. Theranostics. 2018. PMID: 29507617 Free PMC article.
-
Survival motor neuron protein deficiency impairs myotube formation by altering myogenic gene expression and focal adhesion dynamics.Hum Mol Genet. 2014 Sep 15;23(18):4745-57. doi: 10.1093/hmg/ddu189. Epub 2014 Apr 23. Hum Mol Genet. 2014. PMID: 24760765 Free PMC article.
-
The Clinicopathological Distinction between Immune-Mediated Necrotizing Myopathy and Limb-Girdle Muscular Dystrophy R2: Key Points to Prevent Misdiagnosis.J Clin Med. 2022 Nov 5;11(21):6566. doi: 10.3390/jcm11216566. J Clin Med. 2022. PMID: 36362794 Free PMC article.
-
Faster regeneration associated to high expression of Fam65b and Hdac6 in dysferlin-deficient mouse.J Mol Histol. 2019 Aug;50(4):375-387. doi: 10.1007/s10735-019-09834-y. Epub 2019 Jun 19. J Mol Histol. 2019. PMID: 31218594
References
-
- Liu J, Aoki M, Illa I, et al. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet. 1998;20:31–6. - PubMed
-
- Mahjneh I, Marconi G, Bushby K, Anderson LV, Tolvanen-Mahjneh H, Somer H. Dysferlinopathy (LGMD2B): a 23-year follow-up study of 10 patients homozygous for the same frameshifting dysferlin mutations. Neuromuscul Disord. 2001;11:20–6. - PubMed
-
- Bashir R, Strachan T, Keers S, et al. A gene for autosomal recessive limb-girdle muscular dystrophy maps to chromosome 2p. Hum Mol Genet. 1994;3:455–7. - PubMed
-
- Bushby KM. Making sense of the limb-girdle muscular dystrophies. Brain. 1999;122(Pt. 8):1403–20. - PubMed
-
- Linssen WH, Notermans NC, Van der Graaf Y, et al. Miyoshi-type distal muscular dystrophy. Clinical spectrum in 24 Dutch patients. Brain. 1997;120(Pt. 11):1989–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

