Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects
- PMID: 22561291
- PMCID: PMC3810007
- DOI: 10.1016/j.yrtph.2012.04.008
Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects
Abstract
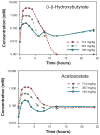
Induction of mild states of hyperketonemia may improve physical and cognitive performance. In this study, we determined the kinetic parameters, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate, a ketone monoester administered in the form of a meal replacement drink to healthy human volunteers. Plasma levels of β-hydroxybutyrate and acetoacetate were elevated following administration of a single dose of the ketone monoester, whether at 140, 357, or 714 mg/kg body weight, while the intact ester was not detected. Maximum plasma levels of ketones were attained within 1-2h, reaching 3.30 mM and 1.19 mM for β-hydroxybutyrate and acetoacetate, respectively, at the highest dose tested. The elimination half-life ranged from 0.8-3.1h for β-hydroxybutyrate and 8-14 h for acetoacetate. The ketone monoester was also administered at 140, 357, and 714 mg/kg body weight, three times daily, over 5 days (equivalent to 0.42, 1.07, and 2.14 g/kg/d). The ketone ester was generally well-tolerated, although some gastrointestinal effects were reported, when large volumes of milk-based drink were consumed, at the highest ketone monoester dose. Together, these results suggest ingestion of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate is a safe and simple method to elevate blood ketone levels, compared with the inconvenience of preparing and consuming a ketogenic diet.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
The intellectual property covering the uses of ketone bodies and ketone esters are owned by BTG Ltd., the University of Oxford and the National Institutes of Health. Should royalties ever accrue from these patents, Dr. Richard L. Veech, Professor Kieran Clarke, and Mr. Todd King, as inventors, will receive a share of the royalties under the terms prescribed by each institution. Professor Kieran Clarke is a non-executive director of TdeltaS Ltd., a company spun out of the University of Oxford to develop products based on the science of ketone bodies in human nutrition. Drs Kathy Musa- Veloso, Manki Ho, and Ashley Roberts received financial support from DARPA and TdeltaS Limited for consulting services and manuscript preparation.
Figures
References
-
- Anders MW. Biotransformation and bioactivation of xenobiotics by the kidney. In: Hutson D, Caldwell J, Paulson GD, editors. Intermediary Xenobiotic Metabolism in Animals: Methodology, Mechanisms and Significance. Taylor and Francis; New York: 1989. pp. 81–97.
-
- Balasse EO, Féry F. Ketone body production and disposal: effects of fasting, diabetes, and exercise. Diabetes Metab Rev. 1989;5:247–270. (abstract only) - PubMed
-
- Barac-Nieto M. Renal hydroxybutyrate and acetoacetate reabsorption and utilization in the rat. Am J Physiol. 1985;249:F40–F48. - PubMed
-
- Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical