Cardiac mTOR protects the heart against ischemia-reperfusion injury
- PMID: 22561297
- PMCID: PMC3404649
- DOI: 10.1152/ajpheart.00241.2012
Cardiac mTOR protects the heart against ischemia-reperfusion injury
Abstract
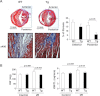
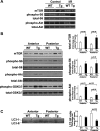
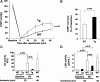
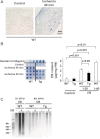
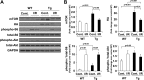
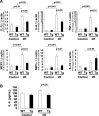
Cardiac mammalian target of rapamycin (mTOR) is necessary and sufficient to prevent cardiac dysfunction in pathological hypertrophy. However, the role of cardiac mTOR in heart failure after ischemic injury remains undefined. To address this question, we used transgenic (Tg) mice with cardiac-specific overexpression of mTOR (mTOR-Tg mice) to study ischemia-reperfusion (I/R) injury in two animal models: 1) in vivo I/R injury with transient coronary artery ligation and 2) ex vivo I/R injury in Langendorff-perfused hearts with transient global ischemia. At 28 days after I/R, mortality was lower in mTOR-Tg mice than littermate control mice [wild-type (WT) mice]. Echocardiography and MRI demonstrated that global cardiac function in mTOR-Tg mice was preserved, whereas WT mice exhibited significant cardiac dysfunction. Masson's trichrome staining showed that 28 days after I/R, the area of interstitial fibrosis was smaller in mTOR-Tg mice compared with WT mice, suggesting that adverse left ventricular remodeling is inhibited in mTOR-Tg mice. In the ex vivo I/R model, mTOR-Tg hearts demonstrated improved functional recovery compared with WT hearts. Perfusion with Evans blue after ex vivo I/R yielded less staining in mTOR-Tg hearts than WT hearts, indicating that mTOR overexpression inhibited necrosis during I/R injury. Expression of proinflammatory cytokines, including IL-6 and TNF-α, in mTOR-Tg hearts was lower than in WT hearts. Consistent with this, IL-6 in the effluent post-I/R injury was lower in mTOR-Tg hearts than in WT hearts. These findings suggest that cardiac mTOR overexpression in the heart is sufficient to provide substantial cardioprotection against I/R injury and suppress the inflammatory response.
Figures

References
-
- Avruch J, Hara K, Lin Y, Liu M, Long X, Ortiz-Vega S, Yonezawa K. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene 25: 6361–6372, 2006 - PubMed
-
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434: 658–662, 2005 - PubMed
-
- Buss SJ, Muenz S, Riffel JH, Malekar P, Hagenmueller M, Weiss CS, Bea F, Bekeredjian R, Schinke-Braun M, Izumo S, Katus HA, Hardt SE. Beneficial effects of Mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol 54: 2435–2446, 2009 - PubMed
-
- Cittadini A, Monti MG, Petrillo V, Esposito G, Imparato G, Luciani A, Urciuolo F, Bobbio E, Natale CF, Sacca L, Netti PA. Complementary therapeutic effects of dual delivery of insulin-like growth factor-1 and vascular endothelial growth factor by gelatin microspheres in experimental heart failure. Eur J Heart Fail 13: 1264–1274, 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

