Lymphocyte-polarized dendritic cells are highly effective in inducing tumor-specific CTLs
- PMID: 22561311
- PMCID: PMC3434244
- DOI: 10.1016/j.vaccine.2012.04.077
Lymphocyte-polarized dendritic cells are highly effective in inducing tumor-specific CTLs
Abstract
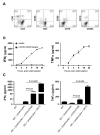
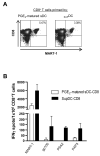
High activity of dendritic cells (DCs) in inducing cytotoxic T cells (CTLs) led to their application as therapeutic cancer vaccines. The ability of DCs to produce IL-12p70 is one of the key requirements for effective CTL induction and a predictive marker of their therapeutic efficacy in vivo. We have previously reported that defined cocktails of cytokines, involving TNFα and IFNγ, induce mature type-1 polarized DCs (DC1s) which produce strongly elevated levels of IL-12 and CXCL10/IP10 upon CD40 ligation compared to "standard" PGE₂-matured DCs (sDCs; matured with IL-1β, IL-6, TNFα, and PGE₂) and show higher CTL-inducing activity. Guided by our observations that DC1s can be induced by TNFα- and IFNγ-producing CD8⁺ T cells, we have tested the feasibility of using lymphocytes to generate DC1s in a clinically-compatible process, to limit the need for clinical-grade recombinant cytokines and the associated costs. CD3/CD28 activation of bulk lymphocytes expanded them and primed them for effective production of IFNγ and TNFα following restimulation. Restimulated lymphocytes, or their culture supernatants, enhanced the maturation status of immature (i)DCs, elevating their expression of CD80, CD83 and CCR7, and the ability to produce IL-12p70 and CXCL10 upon subsequent CD40 ligation. The "lymphocyte-matured" DC1s showed elevated migration in response to the lymph-node-directing chemokine, CCL21, when compared to iDCs. When loaded with antigenic peptides, supernatant-matured DCs induced much high levels of CTLs recognizing tumor-associated antigenic epitope, than PGE₂-matured DCs from the same donors. These results demonstrate the feasibility of generation of polarized DC1s using autologous lymphocytes.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures



References
-
- Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001 Aug 10;106(3):263–266. - PubMed
-
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. - PubMed
-
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. - PubMed
-
- Nestle FO, Filgueira L, Nickoloff BJ, Burg G. Human dermal dendritic cells process and present soluble protein antigens. J Invest Dermatol. 1998 May;110(5):762–766. - PubMed
-
- Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999 Dec;20(12):561–567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

