Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant adenovirus HIV vaccine (Step Study)
- PMID: 22561365
- PMCID: PMC3490694
- DOI: 10.1093/infdis/jis342
Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant adenovirus HIV vaccine (Step Study)
Abstract
Background: The Step Study tested whether an adenovirus serotype 5 (Ad5)-vectored human immunodeficiency virus (HIV) vaccine could prevent HIV acquisition and/or reduce viral load set-point after infection. At the first interim analysis, nonefficacy criteria were met. Vaccinations were halted; participants were unblinded. In post hoc analyses, more HIV infections occurred in vaccinees vs placebo recipients in men who had Ad5-neutralizing antibodies and/or were uncircumcised. Follow-up was extended to assess relative risk of HIV acquisition in vaccinees vs placebo recipients over time.
Methods: We used Cox proportional hazard models for analyses of vaccine effect on HIV acquisition and vaccine effect modifiers, and nonparametric and semiparametric methods for analysis of constancy of relative risk over time.
Results: One hundred seventy-two of 1836 men were infected. The adjusted vaccinees vs placebo recipients hazard ratio (HR) for all follow-up time was 1.40 (95% confidence interval [CI], 1.03-1.92; P= .03). Vaccine effect differed by baseline Ad5 or circumcision status during first 18 months, but neither was significant for all follow-up time. The HR among uncircumcised and/or Ad5-seropositive men waned with time since vaccination. No significant vaccine-associated risk was seen among circumcised, Ad5-negative men (HR, 0.97; P=1.0) over all follow-up time.
Conclusions: The vaccine-associated risk seen in interim analysis was confirmed but waned with time from vaccination.
Trial registration: ClinicalTrials.gov NCT00095576.
Figures


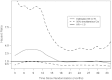
Comment in
-
Reply to Richie and Villasante.J Infect Dis. 2013 Feb 15;207(4):690-2. doi: 10.1093/infdis/jis738. Epub 2012 Nov 29. J Infect Dis. 2013. PMID: 23204171 Free PMC article. No abstract available.
-
Use of adenovirus serotype 5 vaccine vectors in seropositive, uncircumcised men: safety lessons from the step trial.J Infect Dis. 2013 Feb 15;207(4):689-90. doi: 10.1093/infdis/jis737. Epub 2012 Nov 29. J Infect Dis. 2013. PMID: 23204174 No abstract available.
References
-
- Shiver JW, Fu TM, Chen L, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–5. - PubMed
-
- Lu X, Tsiatis AA. Improving the efficiency of the log-rank test using auxiliary covariates. Biometrika. 2008;95:679–94.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

