Functional identification of pathogenic autoantibody responses in patients with multiple sclerosis
- PMID: 22561643
- PMCID: PMC3359756
- DOI: 10.1093/brain/aws105
Functional identification of pathogenic autoantibody responses in patients with multiple sclerosis
Abstract
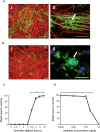
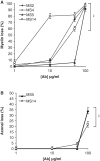
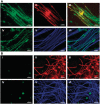
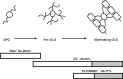
Pathological and clinical studies implicate antibody-dependent mechanisms in the immunopathogenesis of multiple sclerosis. We tested this hypothesis directly by investigating the ability of patient-derived immunoglobulins to mediate demyelination and axonal injury in vitro. Using a myelinating culture system, we developed a sensitive and reproducible bioassay to detect and quantify these effects and applied this to investigate the pathogenic potential of immunoglobulin G preparations obtained from patients with multiple sclerosis (n = 37), other neurological diseases (n = 10) and healthy control donors (n = 13). This identified complement-dependent demyelinating immunoglobulin G responses in approximately 30% of patients with multiple sclerosis, which in two cases was accompanied by significant complement-dependent antibody mediated axonal loss. No pathogenic immunoglobulin G responses were detected in patients with other neurological disease or healthy controls, indicating that the presence of these demyelinating/axopathic autoantibodies is specific for a subset of patients with multiple sclerosis. Immunofluorescence microscopy revealed immunoglobulin G preparations with demyelinating activity contained antibodies that specifically decorated the surface of myelinating oligodendrocytes and their contiguous myelin sheaths. No other binding was observed indicating that the response is restricted to autoantigens expressed by terminally differentiated myelinating oligodendrocytes. In conclusion, our study identifies axopathic and/or demyelinating autoantibody responses in a subset of patients with multiple sclerosis. This observation underlines the mechanistic heterogeneity of multiple sclerosis and provides a rational explanation why some patients benefit from antibody depleting treatments.
Figures

References
-
- Archelos JJ, Hartung HP. Pathogenetic role of autoantibodies in neurological diseases. Trends Neurosci. 2000;23:317–27. - PubMed
-
- Barnett MH, Parratt JD, Cho ES, Prineas JW. Immunoglobulins and complement in postmortem multiple sclerosis tissue. Ann Neurol. 2009;65:32–46. - PubMed
-
- Bradl M, Misu T, Takahashi T, Watanabe M, Mader S, Reindl M, et al. Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Ann Neurol. 2009;66:630–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

