Retinaldehyde dehydrogenase 1 regulates a thermogenic program in white adipose tissue
- PMID: 22561685
- PMCID: PMC3792792
- DOI: 10.1038/nm.2757
Retinaldehyde dehydrogenase 1 regulates a thermogenic program in white adipose tissue
Abstract
Promoting brown adipose tissue (BAT) formation and function may reduce obesity. Recent data link retinoids to energy balance, but a specific role for retinoid metabolism in white versus brown fat is unknown. Retinaldehyde dehydrogenases (Aldhs), also known as aldehyde dehydrogenases, are rate-limiting enzymes that convert retinaldehyde (Rald) to retinoic acid. Here we show that Aldh1a1 is expressed predominately in white adipose tissue (WAT), including visceral depots in mice and humans. Deficiency of the Aldh1a1 gene induced a BAT-like transcriptional program in WAT that drove uncoupled respiration and adaptive thermogenesis. WAT-selective Aldh1a1 knockdown conferred this BAT program in obese mice, limiting weight gain and improving glucose homeostasis. Rald induced uncoupling protein-1 (Ucp1) mRNA and protein levels in white adipocytes by selectively activating the retinoic acid receptor (RAR), recruiting the coactivator PGC-1α and inducing Ucp1 promoter activity. These data establish Aldh1a1 and its substrate Rald as previously unrecognized determinants of adipocyte plasticity and adaptive thermogenesis, which may have potential therapeutic implications.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
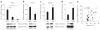


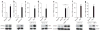

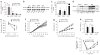
References
-
- Mensah GA, et al. Obesity, metabolic syndrome, and type 2 diabetes: emerging epidemics and their cardiovascular implications. Cardiol Clin. 2004;22:485–504. - PubMed
-
- Bray GA, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine. 2006;29:109–117. - PubMed
-
- Klein S, et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America’s health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Diabetes Care. 2007;30:1647–1652. - PubMed
-
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK048873-14S2/DK/NIDDK NIH HHS/United States
- R01 DK056626/DK/NIDDK NIH HHS/United States
- R01 AR054604/AR/NIAMS NIH HHS/United States
- 5P30DK057521-12/DK/NIDDK NIH HHS/United States
- R37 DK048873/DK/NIDDK NIH HHS/United States
- AR054604-03S1/AR/NIAMS NIH HHS/United States
- R01 DK048873/DK/NIDDK NIH HHS/United States
- P30 DK057521/DK/NIDDK NIH HHS/United States
- DK056626/DK/NIDDK NIH HHS/United States
- DK048873/DK/NIDDK NIH HHS/United States
- R01 DK082659/DK/NIDDK NIH HHS/United States
- J 3107/FWF_/Austrian Science Fund FWF/Austria
- P01 HL048743/HL/NHLBI NIH HHS/United States
- HL048743/HL/NHLBI NIH HHS/United States
- R29 DK048873/DK/NIDDK NIH HHS/United States
- DK082659/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

