T cell homing to epithelial barriers in allergic disease
- PMID: 22561834
- PMCID: PMC3863331
- DOI: 10.1038/nm.2760
T cell homing to epithelial barriers in allergic disease
Abstract
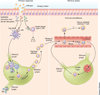
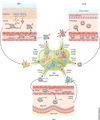
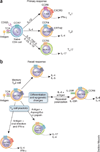
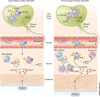
Allergic inflammation develops in tissues that have large epithelial surface areas that are exposed to the environment, such as the lung, skin and gut. In the steady state, antigen-experienced memory T cells patrol these peripheral tissues to facilitate swift immune responses against invading pathogens. In at least two allergy-prone organs, the skin and the gut, memory T cells are programmed during the initial antigen priming to express trafficking receptors that enable them to preferentially home to these organs. In this review we propose that tissue-specific memory and inflammation-specific T cell trafficking facilitates the development of allergic disease in these organs. We thus review recent advances in our understanding of tissue-specific T cell trafficking and how regulation of T cell trafficking by the chemokine system contributes to allergic inflammation in mouse models and in human allergic diseases of the skin, lung and gut. Inflammation- and tissue-specific T lymphocyte trafficking pathways are currently being targeted as new treatments for non-allergic inflammatory diseases and may yield effective new therapeutics for allergic diseases.
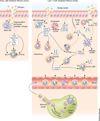
Figures
References
-
- Cookson W. The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat. Rev. Immunol. 2004;4:978–988. - PubMed
-
- Rodriguez E, et al. Meta-analysis of filaggrin polymorphisms in eczema and asthma: robust risk factors in atopic disease. J. Allergy Clin. Immunol. 2009;123:1361–1370. - PubMed
-
- Eiwegger T, Akdis CA. IL-33 links tissue cells, dendritic cells and Th2 cell development in a mouse model of asthma. Eur. J. Immunol. 2011;41:1535–1538. - PubMed
-
- Fort MM, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologiesin vivo . Immunity. 2001;15:985–995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

