Relating micromechanical properties and mineral densities in severely suppressed bone turnover patients, osteoporotic patients, and normal subjects
- PMID: 22561877
- PMCID: PMC4171130
- DOI: 10.1016/j.bone.2012.04.010
Relating micromechanical properties and mineral densities in severely suppressed bone turnover patients, osteoporotic patients, and normal subjects
Abstract
Mineralization of bone, from the tissue level to whole bones, is associated with mechanical properties. The relationship between bone tissue mineralization and micromechanical properties may be affected by age, disease, and drug treatment. Patients with severely suppressed bone turnover (SSBT) suffered atypical fractures while on bisphosphonate treatment. The role of tissue level mineralization in predicting material level properties of SSBT bone may be different from that of other osteoporotic patients and of normal subjects. The aim of this study was to compare the relationships between mineralization and micromechanical properties of bone biopsies from patients with SSBT, bisphosphonate-naive osteoporotic patients with typical vertebral fracture, and normal young and age-matched subjects. We used nanoindentation and quantitative backscattered electron microscopy to characterize the elastic modulus, contact hardness, plastic deformation resistance, and tissue mineralization of the biopsies at site-matched locations within each biopsy. The linear mineralization-mechanical property relationships were different among the groups with respect to the intercepts for only cortical bone tissue but not the slopes for cortical and trabecular bone tissues. For a given mineral density, there was a trend of greater plastic deformation resistance in SSBT cortical bone compared to young normal bone. Similarly, there was a trend of greater plastic deformation resistance in osteoporotic trabecular bone compared to young normal bone for a given mineral density. The age-matched normal group had higher elastic modulus and a trend of higher contact hardness compared to the young normal group for a given mineral density. However, the mechanical property-mineralization relationships within an individual were weak, and only 21 of 53 biopsies that were analyzed had at least one significant association between mineralization and a mechanical property measurement for either cortical or trabecular bone tissues. The average properties of microstructural regions (deep and superficial remodeling packets in trabecular bone; osteonal and interstitial regions in cortical bone) were consistent with mineral accumulation with tissue age, with the exception of the SSBT group. SSBT trabecular bone deep packets had higher hardness and plastic deformation resistance than superficial packets, but mineralization levels and tissue modulus were not different between packet types. We conclude that relationships between mineral and mechanical properties were different between fracture and normal groups and between young and old normal groups, and that atypical fracture may be associated with changed microstructural material properties and tissue level mineralization compared to osteoporotic patients with vertebral fracture and normal subjects. We hypothesize that tissue level bone quality may be an important determinant in fracture risk, such that tissue mineral density may predict different material properties in different patient groups.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


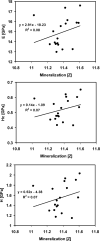


References
-
- Gupta HS, Stachewicz U, Wagermaier W, Roschger P, Wagner HD, Fratzl P. Mechanical modulation at the lamellar level in osteonal bone. Journal of Materials Research. 2006;21:1913–1921.
-
- Fratzl P, Gupta HS, Fischer FD, Kolednik O. Hindered crack propagation in materials with periodically varying Young’s modulus – Lessons from biological materials. Advanced Materials. 2007;19:2657–+.
-
- Currey JD. Effects of differences in mineralization on the mechanical properties of bone. Philos Trans R Soc Lond B Biol Sci. 1984;304:509–18. - PubMed
-
- Oyen ML, Ferguson VL, Bembey AK, Bushby AJ, Boyde A. Composite bounds on the elastic modulus of bone. J Biomech. 2008;41:2585–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

