External validation of the Prostate Cancer Prevention Trial and the European Randomized Study of Screening for Prostate Cancer risk calculators in a Chinese cohort
- PMID: 22561907
- PMCID: PMC3734979
- DOI: 10.1038/aja.2012.28
External validation of the Prostate Cancer Prevention Trial and the European Randomized Study of Screening for Prostate Cancer risk calculators in a Chinese cohort
Abstract
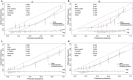
Several prediction models have been developed to estimate the outcomes of prostate biopsies. Most of these tools were designed for use with Western populations and have not been validated across different ethnic groups. Therefore, we evaluated the predictive value of the Prostate Cancer Prevention Trial (PCPT) and the European Randomized Study of Screening for Prostate Cancer (ERSPC) risk calculators in a Chinese cohort. Clinicopathological information was obtained from 495 Chinese men who had undergone extended prostate biopsies between January 2009 and March 2011. The estimated probabilities of prostate cancer and high-grade disease (Gleason >6) were calculated using the PCPT and ERSPC risk calculators. Overall measures, discrimination, calibration and clinical usefulness were assessed for the model evaluation. Of these patients, 28.7% were diagnosed with prostate cancer and 19.4% had high-grade disease. Compared to the PCPT model and the prostate-specific antigen (PSA) threshold of 4 ng ml(-1), the ERSPC risk calculator exhibited better discriminative ability for predicting positive biopsies and high-grade disease (the area under the curve was 0.831 and 0.852, respectively, P<0.01 for both). Decision curve analysis also suggested the favourable clinical utility of the ERSPC calculator in the validation dataset. Both prediction models demonstrated miscalibration: the risk of prostate cancer and high-grade disease was overestimated by approximately 20% for a wide range of predicted probabilities. In conclusion, the ERSPC risk calculator outperformed both the PCPT model and the PSA threshold of 4 ng ml(-1) in predicting prostate cancer and high-grade disease in Chinese patients. However, the prediction tools derived from Western men significantly overestimated the probability of prostate cancer and high-grade disease compared to the outcomes of biopsies in a Chinese cohort.
Figures


References
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 Lyon; International Agency for Research on Cancer; 2010http://globocan.iarc.fr (accessed on 11 August 2011).
-
- Etzioni R, Penson DF, Legler JM, di Tommaso D, Boer R, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94:981–90. - PubMed
-
- Freedland SJ, Isaacs WB. Explaining racial differences in prostate cancer in the United States: sociology or biology. Prostate. 2005;62:243–52. - PubMed
-
- Punglia RS, D'Amico AV, Catalona WJ, Roehl KA, Kuntz KM. Effect of verification bias on screening for prostate cancer by measurement of prostate-specific antigen. N Engl J Med. 2003;349:335–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

