Residue Ile89 in human plasma membrane monoamine transporter influences its organic cation transport activity and sensitivity to inhibition by dilazep
- PMID: 22562044
- PMCID: PMC3372611
- DOI: 10.1016/j.bcp.2012.04.018
Residue Ile89 in human plasma membrane monoamine transporter influences its organic cation transport activity and sensitivity to inhibition by dilazep
Abstract
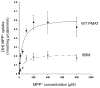
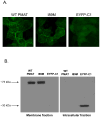
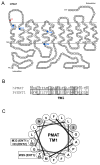
Plasma membrane monoamine transporter (PMAT) is a polyspecific organic cation transporter belonging to the equilibrative nucleoside transporter (ENT) family. Despite its distinct substrate specificity from the classic nucleoside transporters ENT1 and 2, PMAT appears to share similar protein architecture with ENT1/2 and retains low affinity binding to classic ENT inhibitors such as nitrobenzylmercaptopurine riboside (NBMPR) and the coronary vasodilators dilazep and dipyridamole. Here we investigated the role of residue Ile89, a position known to be important for ENT interaction with dilazep, dipyridamole, and nucleoside substrates, in PMAT transport function and its interaction with classic ENT inhibitors using Madin-Darby canine kidney (MDCK) cells stably expressing human PMAT. Substitution of Ile89 in PMAT with Met, the counterpart residue in ENT1, resulted in normal plasma membrane localization and protein expression. Transport kinetic analysis revealed that I89M mutant had a 2.7-fold reduction in maximal transport velocity (V(max)) with no significant change in apparent binding affinity (K(m)) towards the prototype PMAT substrate 1-methyl-4-phenylpyridinium (MPP+), suggesting that I89 is an important determinant for the catalytic activity of PMAT. Dose-dependent inhibition studies further showed that the I89M mutation significantly increased PMAT's sensitivity to dilazep by 2.5-fold without affecting its sensitivity to dipyridamole and NBMPR. Located at the extracellular end of transmembrane domain 1 of PMAT, I89 may occupy an important position close to the substrate permeation pathway and may be involved in direct interaction with the vasodilator dilazep.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004;447:735–43. - PubMed
-
- Kong W, Engel K, Wang J. Mammalian nucleoside transporters. Curr Drug Metab. 2004;5:63–84. - PubMed
-
- Baldwin SA, Yao SY, Hyde RJ, Ng AM, Foppolo S, Barnes K, et al. Functional characterization of novel human and mouse equilibrative nucleoside transporters (hENT3 and mENT3) located in intracellular membranes. J Biol Chem. 2005;280:15880–7. - PubMed
-
- Engel K, Zhou M, Wang J. Identification and characterization of a novel monoamine transporter in the human brain. J Biol Chem. 2004;279:50042–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

