Human PXR-mediated induction of intestinal CYP3A4 attenuates 1α,25-dihydroxyvitamin D₃ function in human colon adenocarcinoma LS180 cells
- PMID: 22562045
- PMCID: PMC3372629
- DOI: 10.1016/j.bcp.2012.04.019
Human PXR-mediated induction of intestinal CYP3A4 attenuates 1α,25-dihydroxyvitamin D₃ function in human colon adenocarcinoma LS180 cells
Abstract
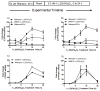
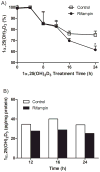
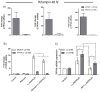
Oxidative catabolism of 1α,25-dihydroxyvitamin D(3) [1α,25(OH)(2)D(3)] is mediated by either CYP24A1 or CYP3A4. In this paper, we tested whether induction of CYP3A4 in the LS180 intestinal cell model enhances clearance of 1α,25(OH)(2)D(3) and blunts its hormonal effect on expression of the apical membrane calcium transport protein, TRPV6. Treatment with the hPXR agonist rifampin significantly increased CYP3A4 mRNA content and catalytic activity, but had no effect on CYP24A1 or TRPV6 mRNA content. Pre-treating cells with rifampin for 48h, prior to a 24h 1α,25(OH)(2)D(3) treatment phase, was associated with a subsequent 48% increase in the elimination of 1α,25(OH)(2)D(3) and a 35% reduction of peak TRPV6 mRNA. Introduction of the CYP3A4 inhibitor, 6',7'-dihydroxybergamottin, an active inhibitor in grapefruit juice, reversed the effects of rifampin on 1α,25(OH)(2)D(3) clearance and TRPV6 expression. Over-expression of hPXR in LS180 cells greatly enhanced the CYP3A4 responsiveness to rifampin pretreatment, and elicited a greater relative suppression of TRPV6 expression and an increase in 1α,25(OH)(2)D(3) disappearance rate, compared to vector expressed cells, following hormone administration. Together, these results suggest that induction of CYP3A4 in the intestinal epithelium by hPXR agonists can result in a greater metabolic clearance of 1α,25(OH)(2)D(3) and reduced effects of the hormone on the intestinal calcium absorption, which may contribute to an increased risk of drug-induced osteomalacia/osteoporosis in patients receiving chronic therapy with potent hPXR agonists. Moreover, ingestion of grapefruit juice in the at-risk patients could potentially prevent this adverse drug effect.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
-
- Brodie MJ, Boobis AR, Dollery CT, Hillyard CJ, Brown DJ, MacIntyre I, et al. Rifampicin and vitamin D metabolism. Clin Pharmacol Ther. 1980;27:810–4. - PubMed
-
- Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5 (Suppl 2):S24–9. - PubMed
-
- Petty SJ, O’Brien TJ, Wark JD. Anti-epileptic medication and bone health. Osteoporos Int. 2007;18:129–42. - PubMed
-
- Khanal RC, Nemere I. Regulation of intestinal calcium transport. Annu Rev Nutr. 2008;28:179–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

