Regulation of the Golgi complex by phospholipid remodeling enzymes
- PMID: 22562055
- PMCID: PMC3399269
- DOI: 10.1016/j.bbalip.2012.04.004
Regulation of the Golgi complex by phospholipid remodeling enzymes
Abstract
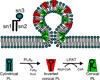
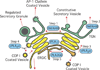
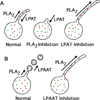
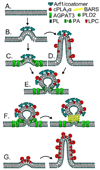
The mammalian Golgi complex is a highly dynamic organelle consisting of stacks of flattened cisternae with associated coated vesicles and membrane tubules that contribute to cargo import and export, intra-cisternal trafficking, and overall Golgi architecture. At the morphological level, all of these structures are continuously remodeled to carry out these trafficking functions. Recent advances have shown that continual phospholipid remodeling by phospholipase A (PLA) and lysophospholipid acyltransferase (LPAT) enzymes, which deacylate and reacylate Golgi phospholipids, respectively, contributes to this morphological remodeling. Here we review the identification and characterization of four cytoplasmic PLA enzymes and one integral membrane LPAT that participate in the dynamic functional organization of the Golgi complex, and how some of these enzymes are integrated to determine the relative abundance of COPI vesicle and membrane tubule formation. This article is part of a Special Issue entitled Lipids and Vesicular Transport.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures


References
-
- Lowe M. Structural organization of the Golgi apparatus. Curr Opin Cell Biol. 2011;23:85–93. - PubMed
-
- Polishchuk RS, Capestrano M, Polishchuk EV. Shaping tubular carriers for intracellular membrane transport. FEBS Lett. 2009;583:3847–3856. - PubMed
-
- Wilson C, Venditti R, Rega LR, Colanzi A, D'Angelo G, De Matteis MA. The Golgi apparatus: an organelle with multiple complex functions. Biochem J. 2011;433:1–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

