Uptake routes of tumor-antigen MAGE-A3 by dendritic cells determine priming of naïve T-cell subtypes
- PMID: 22562379
- PMCID: PMC11028545
- DOI: 10.1007/s00262-012-1272-y
Uptake routes of tumor-antigen MAGE-A3 by dendritic cells determine priming of naïve T-cell subtypes
Abstract
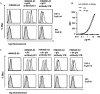
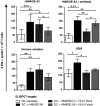
Induction of tumor-antigen-specific T cells in active cancer immunotherapy is generally difficult due to the very low anti-tumoral precursor cytotoxic T cells. By improving tumor-antigen uptake and presentation by dendritic cells (DCs), this problem can be overcome. Focusing on MAGE-A3 protein, frequently expressed in many types of tumors, we analyzed different DC-uptake routes after additional coating the recombinant MAGE-A3 protein with either a specific monoclonal antibody or an immune complex formulation. Opsonization of the protein with antibody resulted in increased DC-uptake compared to the uncoated rhMAGE-A3 protein. This was partly due to Fcγ receptor-dependent internalization. However, unspecific antigen internalization via macropinocytosis also played a role. When analyzing DC-uptake of MAGE-A3 antigen expressed in multiple myeloma cell line U266, pretreatment with proteasome inhibitor bortezomib resulted in increased apoptosis compared to γ-irradiation. Bortezomib-mediated immunogenic apoptosis, characterized by elevated surface expression of hsp90, triggered higher phagocytosis of U266 cells by DCs involving specific DC-derived receptors. We further investigated the impact of antigen delivery on T-cell priming. Induction of CD8(+) T-cell response was favored by stimulating naïve T cells with either antibody-opsonized MAGE-A3 protein or with the bortezomib-pretreated U266 cells, indicating that receptor-mediated uptake favors cross-presentation of antigens. In contrast, CD4(+) T cells were preferentially induced after stimulation with the uncoated protein or protein in the immune complex, both antigen formulations were preferentially internalized by DCs via macropinocytosis. In summary, receptor-mediated DC-uptake mechanisms favored the induction of CD8(+) T cells, relevant for clinical anti-tumor response.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures

 surgery. b Blood was collected after allogeneic PBSCT and stimulated twice with peptide EVDPIGHLY (MAGE-A3/HLA-A1). Mage-A3-specific T-cells were detected by pentamer assay. HCV peptide as negative control
surgery. b Blood was collected after allogeneic PBSCT and stimulated twice with peptide EVDPIGHLY (MAGE-A3/HLA-A1). Mage-A3-specific T-cells were detected by pentamer assay. HCV peptide as negative control
References
-
- Monte M, Simonatto M, Peche LY, Bublik DR, Gobessi S, et al. MAGE-A tumor antigens target p53 transactivation function through histone deacetylase recruitment and confer resistance to chemotherapeutic agents. Proc Natl Acad Sci USA. 2006;103:11160–11165. doi: 10.1073/pnas.0510834103. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

