Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study
- PMID: 22562973
- PMCID: PMC3551224
- DOI: 10.1136/annrheumdis-2011-201247
Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study
Abstract
Objective: To assess the efficacy and safety of adalimumab plus methotrexate (ADA+MTX) compared with methotrexate monotherapy in achieving stable low disease activity (LDA; disease activity score (DAS28(CRP)) <3.2 at weeks 22 and 26) and clinical, radiographic and functional outcomes in methotrexate-naive patients with early rheumatoid arthritis (RA).
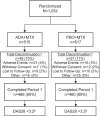
Methods: 1032 patients with active RA were randomly assigned 1:1 to ADA+MTX or placebo plus methotrexate (PBO+MTX) for 26 weeks. Treatment modifications were to be made in a subsequent study period based on the achievement of DAS28(CRP) <3.2 at weeks 22 and 26. Post-hoc analyses compared patients achieving stable remission using DAS28 and 2010 ACR/EULAR criteria with those achieving LDA but not remission.
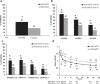
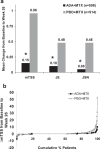
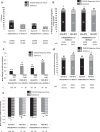
Results: Among patients completing 6 months, 44% (207/466) ADA+MTX versus 24% (112/460) PBO+MTX patients achieved stable LDA at weeks 22 and 26 (p<0.001). Combination therapy was statistically superior to methotrexate in obtaining higher ACR20/50/70 responses, more clinical remissions, greater mean reductions in DAS28(CRP), no radiographic progression, and normal functional status at week 26 (p<0.001 for all). The only factor predicting stable LDA was disease activity at week 12. Patients achieving ACR/EULAR remission, particularly in the PBO+MTX group, had some advantage in radiographic outcomes compared with patients who only achieved LDA (but not remission). The overall frequency of adverse events was comparable between groups. There were more serious infections and deaths in the ADA+MTX group, with a possible age effect.
Conclusions: Treatment with ADA+MTX was significantly superior to methotrexate alone with respect to clinical, radiographic and functional outcomes in patients with early active RA. Before initiating treatment with adalimumab, individual patient evaluation of the benefit/risk ratio should be carefully considered.
Conflict of interest statement
Figures
Comment in
-
[Adalimumab for early rheumatoid arthritis - Chances and risks: Initial analyses of the OPTIMA study].Z Rheumatol. 2013 Aug;72(6):606-7. doi: 10.1007/s00393-013-1184-z. Z Rheumatol. 2013. PMID: 23685856 German. No abstract available.
References
-
- Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008;59:762–84 - PubMed
-
- Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis 2011;70:404–13 - PubMed
-
- Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24 - PubMed
-
- Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727–35 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

