Low-dose prednisone chronotherapy for rheumatoid arthritis: a randomised clinical trial (CAPRA-2)
- PMID: 22562974
- PMCID: PMC3553491
- DOI: 10.1136/annrheumdis-2011-201067
Low-dose prednisone chronotherapy for rheumatoid arthritis: a randomised clinical trial (CAPRA-2)
Abstract
Objective: To assess the efficacy and safety of low-dose prednisone chronotherapy using a new modified-release (MR) formulation for the treatment of rheumatoid arthritis (RA).
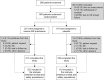
Methods: In this 12-week, double-blind, placebo-controlled study, patients with active RA (n=350) were randomised 2:1 to receive MR prednisone 5 mg or placebo once daily in the evening in addition to their existing RA disease-modifying antirheumatic drug (DMARD) treatment. The primary end point was the percentage of patients achieving a 20% improvement in RA signs and symptoms according to American College of Rheumatology criteria (ie, an ACR20 response) at week 12. Changes in morning pain, duration of morning stiffness, 28-joint Disease Activity Score and health-related quality of life were also assessed.
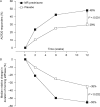
Results: MR prednisone plus DMARD treatment produced higher response rates for ACR20 (48% vs 29%, p<0.001) and ACR50 (22% vs 10%, p<0.006) and a greater median relative reduction from baseline in morning stiffness (55% vs 35%, p<0.002) at week 12 than placebo plus DMARD treatment. Significantly greater reductions in severity of RA (Disease Activity Score 28) (p<0.001) and fatigue (Functional Assessment of Chronic Illness Therapy-Fatigue score) (p=0.003) as well as a greater improvement in physical function (36-item Short-Form Health Survey score) (p<0.001) were seen at week 12 for MR prednisone versus placebo. The incidence of adverse events was similar for MR prednisone (43%) and placebo (49%).
Conclusion: Low-dose MR prednisone added to existing DMARD treatment produced rapid and relevant improvements in RA signs and symptoms. CLINICALTRIALS.GOV, NUMBER: NCT00650078.
Conflict of interest statement
Figures
References
-
- Ezeugo U, Glasser SP. Clinical benefits versus shortcomings of diltiazem once-daily in the chronotherapy of cardiovascular diseases. Expert Opin Pharmacother 2009;10:485–91 - PubMed

