Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY)
- PMID: 22562983
- PMCID: PMC3551223
- DOI: 10.1136/annrheumdis-2011-201282
Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY)
Abstract
Objective: In patients with active rheumatoid arthritis (RA) despite methotrexate, to compare the efficacy of adding tocilizumab to that of switching to tocilizumab monotherapy.
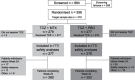
Methods: Double-blind, 2-year study in which adults with active RA (DAS28 >4.4) despite methotrexate were randomly assigned either to continue methotrexate with the addition of tocilizumab (MTX+TCZ) 8 mg/kg every 4 weeks or switch to tocilizumab and placebo (TCZ+PBO). The primary endpoint was the DAS28-erythrocyte sedimentation rate (ESR) remission rate at week 24. Secondary objectives included other symptomatic outcomes, quality of life and progression of structural damage.
Results: Of 556 randomly assigned patients, 512 (92%) completed 24 weeks. DAS28-ESR remission rates were 40.4% for TCZ+MTX and 34.8% for TCZ+PBO (p=0.19); American College of Rheumatology 20/50/70/90 rates were 71.5%/45.5%/24.5%/5.8% (TCZ+MTX) and 70.3%/40.2%/25.4%/5.1% (TCZ+PBO; differences not significant). A significant difference between groups was seen for low DAS28 (61.7% vs 51.4%). Radiographic progression was small and not different between groups (Genant-Sharp score progression ≤ smallest detectable change in 91% (TCZ+MTX) and 87% (TCZ+PBO)). Rates per 100 patient-years of serious adverse events and serious infections were 21 and six, respectively, for TCZ+MTX and 18 and six, respectively, for TCZ+PBO. Alanine aminotransferase elevations greater than threefold the upper limit of normal occurred in 7.8% and 1.2% of TCZ+MTX and TCZ+PBO patients, respectively.
Conclusion: No clinically relevant superiority of the TCZ+MTX add-on strategy over the switch to tocilizumab monotherapy strategy was observed. The combination was more commonly associated with transaminase increases. Meaningful clinical and radiographic responses were achieved with both strategies, suggesting that tocilizumab monotherapy might be a valuable treatment strategy in suitable RA patients.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

