Export requirements of pneumolysin in Streptococcus pneumoniae
- PMID: 22563048
- PMCID: PMC3393478
- DOI: 10.1128/JB.00114-12
Export requirements of pneumolysin in Streptococcus pneumoniae
Abstract
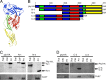
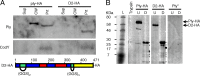
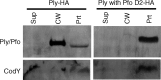
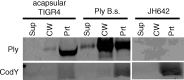
Streptococcus pneumoniae is a major causative agent of otitis media, pneumonia, bacteremia, and meningitis. Pneumolysin (Ply), a member of the cholesterol-dependent cytolysins (CDCs), is produced by virtually all clinical isolates of S. pneumoniae, and ply mutant strains are severely attenuated in mouse models of colonization and infection. In contrast to all other known members of the CDC family, Ply lacks a signal peptide for export outside the cell. Instead, Ply has been hypothesized to be released upon autolysis or, alternatively, via a nonautolytic mechanism that remains undefined. We show that an exogenously added signal sequence is not sufficient for Sec-dependent Ply secretion in S. pneumoniae but is sufficient in the surrogate host Bacillus subtilis. Previously, we showed that Ply is localized primarily to the cell wall compartment in the absence of detectable cell lysis. Here we show that Ply released by autolysis cannot reassociate with intact cells, suggesting that there is a Ply export mechanism that is coupled to cell wall localization of the protein. This putative export mechanism is capable of secreting a related CDC without its signal sequence. We show that B. subtilis can export Ply, suggesting that the export pathway is conserved. Finally, through truncation and domain swapping analyses, we show that export is dependent on domain 2 of Ply.
Figures




References
-
- Bassford P, Beckwith J. 1979. Escherichia coli mutants accumulating the precursor of a secreted protein in the cytoplasm. Nature 277:538–541 - PubMed
-
- Bergmann S, Rohde M, Chhatwal GS, Hammerschmidt S. 2001. α-Enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Mol. Microbiol. 40:1273–1287 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

