Novel mechanism for negatively regulating Rho-kinase (ROCK) signaling through Coronin1B protein in neuregulin 1 (NRG-1)-induced tumor cell motility
- PMID: 22563075
- PMCID: PMC3381146
- DOI: 10.1074/jbc.M112.346114
Novel mechanism for negatively regulating Rho-kinase (ROCK) signaling through Coronin1B protein in neuregulin 1 (NRG-1)-induced tumor cell motility
Abstract
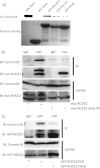
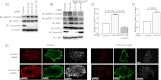
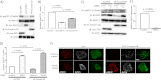
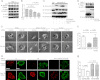
Although many mechanisms that activate ROCK are known, corresponding negative regulatory mechanisms required for cytoskeletal plasticity are poorly understood. We have discovered that Coronin1B is a novel attenuator of ROCK signaling. We initially identified Coronin1A in a proteomics screen for ROCK2-binding proteins, and here we demonstrate that Coronin1A/B bind directly to ROCK2 through its PH (Pleckstrin Homology) domain. The consequence of the ROCK2-Coronin1B interaction was tested and revealed that increased expression of Coronin1B inhibited, whereas knockdown of Coronin1B stimulated, phosphorylation of the ROCK substrate myosin light chain phosphatase and subsequently, myosin light chain. Thus, Coronin1B is a previously unrecognized inhibitor of ROCK signaling to myosin. Furthermore, we found that the phosphatase Slingshot IL (SSH1L) was required for Coronin1B to inhibit ROCK signaling. To test the significance of this novel mechanism in tumor cell motility, we investigated its role in neuregulin 1 (NRG-1)-induced cell scattering. Importantly, we found that attenuation of the ROCK signaling by Coronin1B was required for NRG-1 stimulated scattering. Our data support a model in which Coronin1B fine-tunes ROCK signaling to modulate myosin activity, which is important for tumor cell motility.
Figures



References
-
- Fischer O. M., Hart S., Gschwind A., Ullrich A. (2003) EGFR signal transactivation in cancer cells. Biochem. Soc. Trans. 31, 1203–1208 - PubMed
-
- Gray S. G., Stenfeldt Mathiasen I., De Meyts P. (2003) The insulin-like growth factors and insulin-signaling systems: an appealing target for breast cancer therapy? Horm. Metab. Res. 35, 857–871 - PubMed
-
- Yarden Y., Sliwkowski M. X. (2001) Untangling the ErbB signalling network. Nat. Rev. Mol. Cell. Biol. 2, 127–137 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

