Exercise is more effective than diet control in preventing high fat diet-induced β-amyloid deposition and memory deficit in amyloid precursor protein transgenic mice
- PMID: 22563077
- PMCID: PMC3391129
- DOI: 10.1074/jbc.M112.367011
Exercise is more effective than diet control in preventing high fat diet-induced β-amyloid deposition and memory deficit in amyloid precursor protein transgenic mice
Abstract
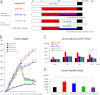
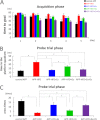
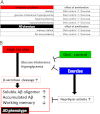
Accumulating evidence suggests that some dietary patterns, specifically high fat diet (HFD), increase the risk of developing sporadic Alzheimer disease (AD). Thus, interventions targeting HFD-induced metabolic dysfunctions may be effective in preventing the development of AD. We previously demonstrated that amyloid precursor protein (APP)-overexpressing transgenic mice fed HFD showed worsening of cognitive function when compared with control APP mice on normal diet. Moreover, we reported that voluntary exercise ameliorates HFD-induced memory impairment and β-amyloid (Aβ) deposition. In the present study, we conducted diet control to ameliorate the metabolic abnormality caused by HFD on APP transgenic mice and compared the effect of diet control on cognitive function with that of voluntary exercise as well as that of combined (diet control plus exercise) treatment. Surprisingly, we found that exercise was more effective than diet control, although both exercise and diet control ameliorated HFD-induced memory deficit and Aβ deposition. The production of Aβ was not different between the exercise- and the diet control-treated mice. On the other hand, exercise specifically strengthened the activity of neprilysin, the Aβ-degrading enzyme, the level of which was significantly correlated with that of deposited Aβ in our mice. Notably, the effect of the combination treatment (exercise and diet control) on memory and amyloid pathology was not significantly different from that of exercise alone. These studies provide solid evidence that exercise is a useful intervention to rescue HFD-induced aggravation of cognitive decline in transgenic model mice of AD.
Figures




References
-
- Finder V. H. (2010) Naturally secreted oligomers of amyloid-β protein potently inhibit hippocampal long-term potentiation in vivo. J. Alzheimers Dis. 22, Suppl. 3, 5–19
-
- Walsh D. M., Klyubin I., Fadeeva J. V., Cullen W. K., Anwyl R., Wolfe M. S., Rowan M. J., Selkoe D. J. (2002) Naturally secreted oligomers of amyloid-β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 - PubMed
-
- Lesné S., Koh M. T., Kotilinek L., Kayed R., Glabe C. G., Yang A., Gallagher M., Ashe K. H. (2006) A specific amyloid-β protein assembly in the brain impairs memory. Nature 440, 352–357 - PubMed
-
- Shankar G. M., Li S., Mehta T. H., Garcia-Munoz A., Shepardson N. E., Smith I., Brett F. M., Farrell M. A., Rowan M. J., Lemere C. A., Regan C. M., Walsh D. M., Sabatini B. L., Selkoe D. J. (2008) Amyloid-β protein dimers isolated directly from Alzheimer brains impair synaptic plasticity and memory. Nat. Med. 14, 837–842 - PMC - PubMed
-
- Luchsinger J. A., Tang M. X., Shea S., Mayeux R. (2002) Caloric intake and the risk of Alzheimer disease. Arch. Neurol. 59, 1258–1263 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

