Requirements for stress granule recruitment of fused in sarcoma (FUS) and TAR DNA-binding protein of 43 kDa (TDP-43)
- PMID: 22563080
- PMCID: PMC3391091
- DOI: 10.1074/jbc.M111.328757
Requirements for stress granule recruitment of fused in sarcoma (FUS) and TAR DNA-binding protein of 43 kDa (TDP-43)
Abstract
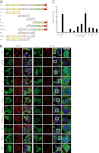
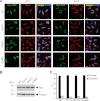
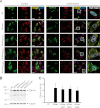
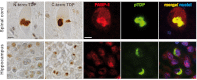
Cytoplasmic inclusions containing TAR DNA-binding protein of 43 kDa (TDP-43) or Fused in sarcoma (FUS) are a hallmark of amyotrophic lateral sclerosis (ALS) and several subtypes of frontotemporal lobar degeneration (FTLD). FUS-positive inclusions in FTLD and ALS patients are consistently co-labeled with stress granule (SG) marker proteins. Whether TDP-43 inclusions contain SG markers is currently still debated. We determined the requirements for SG recruitment of FUS and TDP-43 and found that cytoplasmic mislocalization is a common prerequisite for SG recruitment of FUS and TDP-43. For FUS, the arginine-glycine-glycine zinc finger domain, which is the protein's main RNA binding domain, is most important for SG recruitment, whereas the glycine-rich domain and RNA recognition motif (RRM) domain have a minor contribution and the glutamine-rich domain is dispensable. For TDP-43, both the RRM1 and the C-terminal glycine-rich domain are required for SG localization. ALS-associated point mutations located in the glycine-rich domain of TDP-43 do not affect SG recruitment. Interestingly, a 25-kDa C-terminal fragment of TDP-43, which is enriched in FTLD/ALS cortical inclusions but not spinal cord inclusions, fails to be recruited into SG. Consistently, inclusions in the cortex of FTLD patients, which are enriched for C-terminal fragments, are not co-labeled with the SG marker poly(A)-binding protein 1 (PABP-1), whereas inclusions in spinal cord, which contain full-length TDP-43, are frequently positive for this marker protein.
Figures






References
-
- Mackenzie I. R., Rademakers R., Neumann M. (2010) TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. 9, 995–1007 - PubMed
-
- Buratti E., Baralle F. E. (2008) Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front. Biosci. 13, 867–878 - PubMed
-
- Neumann M., Sampathu D. M., Kwong L. K., Truax A. C., Micsenyi M. C., Chou T. T., Bruce J., Schuck T., Grossman M., Clark C. M., McCluskey L. F., Miller B. L., Masliah E., Mackenzie I. R., Feldman H., Feiden W., Kretzschmar H. A., Trojanowski J. Q., Lee V. M. (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 - PubMed
-
- Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D., Tsuchiya K., Yoshida M., Hashizume Y., Oda T. (2006) TDP-43 is a component of ubiquitin-positive Tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 351, 602–611 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

