An easy α-glycosylation methodology for the synthesis and stereochemistry of mycoplasma α-glycolipid antigens
- PMID: 22563361
- PMCID: PMC3343289
- DOI: 10.3762/bjoc.8.70
An easy α-glycosylation methodology for the synthesis and stereochemistry of mycoplasma α-glycolipid antigens
Abstract
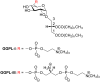
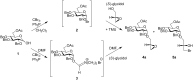
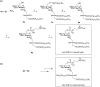
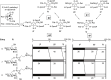
Mycoplasma fermentans possesses unique α-glycolipid antigens (GGPL-I and GGPL-III) at the cytoplasm membrane, which carry a phosphocholine group at the sugar primary (6-OH) position. This paper describes a practical synthetic pathway to a GGPL-I homologue (C(16:0)) and its diastereomer, in which our one-pot α-glycosylation method was effectively applied. The synthetic GGPL-I isomers were characterized with (1)H NMR spectroscopy to determine the equilibrium among the three conformers (gg, gt, tg) at the acyclic glycerol moiety. The natural GGPL-I isomer was found to prefer gt (54%) and gg (39%) conformers around the lipid tail, while adopting all of the three conformers with equal probability around the sugar position. This property was very close to what we have observed with respect to the conformation of phosphatidylcholine (DPPC), suggesting that the Mycoplasma glycolipids GGPLs may constitute the cytoplasm fluid membrane together with ubiquitous phospholipids, without inducing stereochemical stress.
Keywords: cytoplasm membrane; glycolipid antigen; glycosylation; mycoplasma; stereochemistry.
Figures



References
-
- Boggs J M. Biochim Biophys Acta. 1987;906:353–404. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
