Bloom's syndrome and PICH helicases cooperate with topoisomerase IIα in centromere disjunction before anaphase
- PMID: 22563370
- PMCID: PMC3338505
- DOI: 10.1371/journal.pone.0033905
Bloom's syndrome and PICH helicases cooperate with topoisomerase IIα in centromere disjunction before anaphase
Abstract
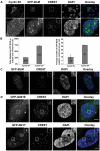
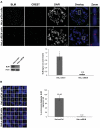
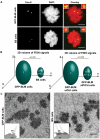
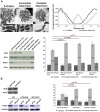
Centromeres are specialized chromosome domains that control chromosome segregation during mitosis, but little is known about the mechanisms underlying the maintenance of their integrity. Centromeric ultrafine anaphase bridges are physiological DNA structures thought to contain unresolved DNA catenations between the centromeres separating during anaphase. BLM and PICH helicases colocalize at these ultrafine anaphase bridges and promote their resolution. As PICH is detectable at centromeres from prometaphase onwards, we hypothesized that BLM might also be located at centromeres and that the two proteins might cooperate to resolve DNA catenations before the onset of anaphase. Using immunofluorescence analyses, we demonstrated the recruitment of BLM to centromeres from G2 phase to mitosis. With a combination of fluorescence in situ hybridization, electron microscopy, RNA interference, chromosome spreads and chromatin immunoprecipitation, we showed that both BLM-deficient and PICH-deficient prometaphase cells displayed changes in centromere structure. These cells also had a higher frequency of centromeric non disjunction in the absence of cohesin, suggesting the persistence of catenations. Both proteins were required for the correct recruitment to the centromere of active topoisomerase IIα, an enzyme specialized in the catenation/decatenation process. These observations reveal the existence of a functional relationship between BLM, PICH and topoisomerase IIα in the centromere decatenation process. They indicate that the higher frequency of centromeric ultrafine anaphase bridges in BLM-deficient cells and in cells treated with topoisomerase IIα inhibitors is probably due not only to unresolved physiological ultrafine anaphase bridges, but also to newly formed ultrafine anaphase bridges. We suggest that BLM and PICH cooperate in rendering centromeric catenates accessible to topoisomerase IIα, thereby facilitating correct centromere disjunction and preventing the formation of supernumerary centromeric ultrafine anaphase bridges.
Conflict of interest statement
Figures

References
-
- Carroll CW, Straight AF. Centromere formation: from epigenetics to self-assembly. Trends Cell Biol. 2006;16:70–78. - PubMed
-
- Cleveland DW, Mao Y, Sullivan KF. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell. 2003;112:407–421. - PubMed
-
- Yuen KW, Montpetit B, Hieter P. The kinetochore and cancer: what’s the connection? Curr Opin Cell Biol. 2005;17:576–582. - PubMed
-
- Baumann C, Korner R, Hofmann K, Nigg EA. PICH, a centromere-associated SNF2 family ATPase, is regulated by Plk1 and required for the spindle checkpoint. Cell. 2007;128:101–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

