Effect of synthetic aβ peptide oligomers and fluorinated solvents on Kv1.3 channel properties and membrane conductance
- PMID: 22563377
- PMCID: PMC3338507
- DOI: 10.1371/journal.pone.0035090
Effect of synthetic aβ peptide oligomers and fluorinated solvents on Kv1.3 channel properties and membrane conductance
Abstract
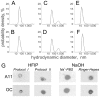
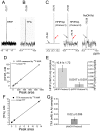
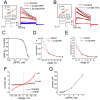
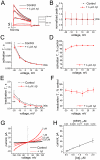
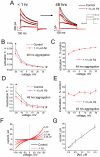
The impact of synthetic amyloid β (1-42) (Aβ(1-42)) oligomers on biophysical properties of voltage-gated potassium channels Kv 1.3 and lipid bilayer membranes (BLMs) was quantified for protocols using hexafluoroisopropanol (HFIP) or sodium hydroxide (NaOH) as solvents prior to initiating the oligomer formation. Regardless of the solvent used Aβ(1-42) samples contained oligomers that reacted with the conformation-specific antibodies A11 and OC and had similar size distributions as determined by dynamic light scattering. Patch-clamp recordings of the potassium currents showed that synthetic Aβ(1-42) oligomers accelerate the activation and inactivation kinetics of Kv 1.3 current with no significant effect on current amplitude. In contrast to oligomeric samples, freshly prepared, presumably monomeric, Aβ(1-42) solutions had no effect on Kv 1.3 channel properties. Aβ(1-42) oligomers had no effect on the steady-state current (at -80 mV) recorded from Kv 1.3-expressing cells but increased the conductance of artificial BLMs in a dose-dependent fashion. Formation of amyloid channels, however, was not observed due to conditions of the experiments. To exclude the effects of HFIP (used to dissolve lyophilized Aβ(1-42) peptide), and trifluoroacetic acid (TFA) (used during Aβ(1-42) synthesis), we determined concentrations of these fluorinated compounds in the stock Aβ(1-42) solutions by (19)F NMR. After extensive evaporation, the concentration of HFIP in the 100× stock Aβ(1-42) solutions was ∼1.7 μM. The concentration of residual TFA in the 70× stock Aβ(1-42) solutions was ∼20 μM. Even at the stock concentrations neither HFIP nor TFA alone had any effect on potassium currents or BLMs. The Aβ(1-42) oligomers prepared with HFIP as solvent, however, were more potent in the electrophysiological tests, suggesting that fluorinated compounds, such as HFIP or structurally-related inhalational anesthetics, may affect Aβ(1-42) aggregation and potentially enhance ability of oligomers to modulate voltage-gated ion channels and biological membrane properties.
Conflict of interest statement
Figures

References
-
- Bertram L, Lill CM, Tanzi RE. The Genetics of Alzheimer Disease: Back to the Future. Neuron. 2010;68:270–281. - PubMed
-
- Chouliaras L, Rutten BPF, Kenis G, Peerbooms O, Visser PJ, et al. Epigenetic regulation in the pathophysiology of Alzheimer's disease. Prog Neurobiol. 2010;90:498–510. - PubMed
-
- Esteban JA. Living with the enemy: a physiological role for the [beta]-amyloid peptide. Trends Neurosci. 2004;27:1–3. - PubMed
-
- Ramsden M, Henderson Z, Pearson HA. Modulation of Ca2+ channel currents in primary cultures of rat cortical neurones by amyloid [beta] protein (1–40) is dependent on solubility status. Brain Res. 2002;956:254–261. - PubMed

