Kidins220/ARMS is a novel modulator of short-term synaptic plasticity in hippocampal GABAergic neurons
- PMID: 22563401
- PMCID: PMC3338529
- DOI: 10.1371/journal.pone.0035785
Kidins220/ARMS is a novel modulator of short-term synaptic plasticity in hippocampal GABAergic neurons
Abstract
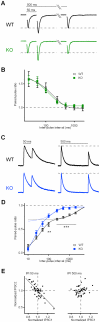
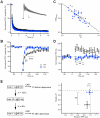
Kidins220 (Kinase D interacting substrate of 220 kDa)/ARMS (Ankyrin Repeat-rich Membrane Spanning) is a scaffold protein highly expressed in the nervous system. Previous work on neurons with altered Kidins220/ARMS expression suggested that this protein plays multiple roles in synaptic function. In this study, we analyzed the effects of Kidins220/ARMS ablation on basal synaptic transmission and on a variety of short-term plasticity paradigms in both excitatory and inhibitory synapses using a recently described Kidins220 full knockout mouse. Hippocampal neuronal cultures prepared from embryonic Kidins220(-/-) (KO) and wild type (WT) littermates were used for whole-cell patch-clamp recordings of spontaneous and evoked synaptic activity. Whereas glutamatergic AMPA receptor-mediated responses were not significantly affected in KO neurons, specific differences were detected in evoked GABAergic transmission. The recovery from synaptic depression of inhibitory post-synaptic currents in WT cells showed biphasic kinetics, both in response to paired-pulse and long-lasting train stimulation, while in KO cells the respective slow components were strongly reduced. We demonstrate that the slow recovery from synaptic depression in WT cells is caused by a transient reduction of the vesicle release probability, which is absent in KO neurons. These results suggest that Kidins220/ARMS is not essential for basal synaptic transmission and various forms of short-term plasticity, but instead plays a novel role in the mechanisms regulating the recovery of synaptic strength in GABAergic synapses.
Conflict of interest statement
Figures




References
-
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. - PubMed
-
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. - PubMed
-
- Gottmann K, Mittmann T, Lessmann V. BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses. Exp Brain Res. 2009;199:203–234. - PubMed
-
- Iglesias T, Cabrera-Poch N, Mitchell MP, Naven TJ, Rozengurt E, et al. Identification and cloning of Kidins220, a novel neuronal substrate of protein kinase D. J Biol Chem. 2000;275:40048–40056. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

