Molecular dynamics simulation of the complex PBP-2x with drug cefuroxime to explore the drug resistance mechanism of Streptococcus suis R61
- PMID: 22563422
- PMCID: PMC3338546
- DOI: 10.1371/journal.pone.0035941
Molecular dynamics simulation of the complex PBP-2x with drug cefuroxime to explore the drug resistance mechanism of Streptococcus suis R61
Abstract
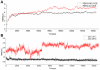
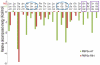
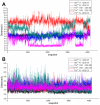
Drug resistance of Streptococcus suis strains is a worldwide problem for both humans and pigs. Previous studies have noted that penicillin-binding protein (PBPs) mutation is one important cause of β-lactam antibiotic resistance. In this study, we used the molecular dynamics (MD) method to study the interaction differences between cefuroxime (CES) and PBP2x within two newly sequenced Streptococcus suis: drug-sensitive strain A7, and drug-resistant strain R61. The MM-PBSA results proved that the drug bound much more tightly to PBP2x in A7 (PBP2x-A7) than to PBP2x in R61 (PBP2x-R61). This is consistent with the evidently different resistances of the two strains to cefuroxime. Hydrogen bond analysis indicated that PBP2x-A7 preferred to bind to cefuroxime rather than to PBP2x-R61. Three stable hydrogen bonds were formed by the drug and PBP2x-A7, while only one unstable bond existed between the drug and PBP2x-R61. Further, we found that the Gln569, Tyr594, and Gly596 residues were the key mutant residues contributing directly to the different binding by pair wise energy decomposition comparison. By investigating the binding mode of the drug, we found that mutant residues Ala320, Gln553, and Thr595 indirectly affected the final phenomenon by topological conformation alteration. Above all, our results revealed some details about the specific interaction between the two PBP2x proteins and the drug cefuroxime. To some degree, this explained the drug resistance mechanism of Streptococcus suis and as a result could be helpful for further drug design or improvement.
Conflict of interest statement
Figures




References
-
- Willenburg KS, Sentochnik DE, Zadoks RN. Human Streptococcus suis meningitis in the United States. N Engl J Med. 2006;354:1325–1325. - PubMed
-
- Wangkaew S, Chaiwarith R, Tharavichitkul P, Supparatpinyo K. Streptococcus suis infection: a series of 41 cases from Chiang Mai University Hospital. Journal of Infection. 2006;52:455–460. - PubMed
-
- Sakata H. Antibiotic susceptibility to parental antibiotics and penicillin-binding-protein genotype in Haemophilus influenzae isolated from children with invasive infection. The Journal of the Japanese Association for Infectious Diseases. 2011;85:26–30. - PubMed
-
- Wilke MS, Lovering AL, Strynadka NCJ. Beta-Lactam antibiotic resistance: a current structural perspective. Current opinion in microbiology. 2005;8:525–533. - PubMed
-
- Martel A, Baele M, Devriese L, Goossens H, Wisselink H, et al. Prevalence and mechanism of resistance against macrolides and lincosamides in Streptococcus suis isolates. Veterinary microbiology. 2001;83:287–297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

