Effects of PPARs agonists on cardiac metabolism in littermate and cardiomyocyte-specific PPAR-γ-knockout (CM-PGKO) mice
- PMID: 22563432
- PMCID: PMC3338561
- DOI: 10.1371/journal.pone.0035999
Effects of PPARs agonists on cardiac metabolism in littermate and cardiomyocyte-specific PPAR-γ-knockout (CM-PGKO) mice
Abstract
Understanding the molecular regulatory mechanisms controlling for myocardial lipid metabolism is of critical importance for the development of new therapeutic strategies for heart diseases. The role of PPARγ and thiazolidinediones in regulation of myocardial lipid metabolism is controversial. The aim of our study was to assess the role of PPARγ on myocardial lipid metabolism and function and differentiate local/from systemic actions of PPARs agonists using cardiomyocyte-specific PPARγ -knockout (CM-PGKO) mice. To this aim, the effect of PPARγ, PPARγ/PPARα and PPARα agonists on cardiac function, intra-myocyte lipid accumulation and myocardial expression profile of genes and proteins, affecting lipid oxidation, uptake, synthesis, and storage (CD36, CPT1MIIA, AOX, FAS, SREBP1-c and ADPR) was evaluated in cardiomyocyte-specific PPARγ-knockout (CM-PGKO) and littermate control mice undergoing standard and high fat diet (HFD). At baseline, protein levels and mRNA expression of genes involved in lipid uptake, oxidation, synthesis, and accumulation of CM-PGKO mice were not significantly different from those of their littermate controls. At baseline, no difference in myocardial lipid content was found between CM-PGKO and littermate controls. In standard condition, pioglitazone and rosiglitazone do not affect myocardial metabolism while, fenofibrate treatment significantly increased CD36 and CPT1MIIA gene expression. In both CM-PGKO and control mice submitted to HFD, six weeks of treatment with rosiglitazone, fenofibrate and pioglitazone lowered myocardial lipid accumulation shifting myocardial substrate utilization towards greater contribution of glucose. In conclusion, at baseline, PPARγ does not play a crucial role in regulating cardiac metabolism in mice, probably due to its low myocardial expression. PPARs agonists, indirectly protect myocardium from lipotoxic damage likely reducing fatty acids delivery to the heart through the actions on adipose tissue. Nevertheless a direct non-PPARγ mediated mechanism of PPARγ agonist could not be ruled out.
Conflict of interest statement
Figures
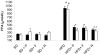
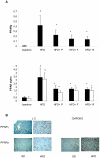

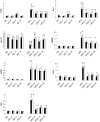
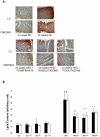
References
-
- Borradaile NM, Schaffer JE. Lipo0toxicity in the heart. Curr Hypertens Rep. 2005;7(6):412–7. - PubMed
-
- McGavock JM, Victor RG, Unger RH, Szczepaniak LS. Adiposity of the heart, revisited. Ann Intern Med. 2006;144:517–524. - PubMed
-
- McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 2007;116:1170–1175. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

