Ghrelin treatment of cachectic patients with chronic obstructive pulmonary disease: a multicenter, randomized, double-blind, placebo-controlled trial
- PMID: 22563468
- PMCID: PMC3341383
- DOI: 10.1371/journal.pone.0035708
Ghrelin treatment of cachectic patients with chronic obstructive pulmonary disease: a multicenter, randomized, double-blind, placebo-controlled trial
Abstract
Background: Pulmonary cachexia is common in advanced chronic obstructive pulmonary disease (COPD), culminating in exercise intolerance and a poor prognosis. Ghrelin is a novel growth hormone (GH)-releasing peptide with GH-independent effects. The efficacy and safety of adding ghrelin to pulmonary rehabilitation (PR) in cachectic COPD patients were investigated.
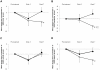
Methodology/principal findings: In a multicenter, randomized, double-blind, placebo-controlled trial, 33 cachectic COPD patients were randomly assigned PR with intravenous ghrelin (2 µg/kg) or placebo twice daily for 3 weeks in hospital. The primary outcomes were changes in 6-min walk distance (6-MWD) and the St. George Respiratory Questionnaire (SGRQ) score. Secondary outcomes included changes in the Medical Research Council (MRC) scale, and respiratory muscle strength. At pre-treatment, serum GH levels were increased from baseline levels by a single dose of ghrelin (mean change, +46.5 ng/ml; between-group p<0.0001), the effect of which continued during the 3-week treatment. In the ghrelin group, the mean change from pre-treatment in 6-MWD was improved at Week 3 (+40 m, within-group p = 0.033) and was maintained at Week 7 (+47 m, within-group p = 0.017), although the difference between ghrelin and placebo was not significant. At Week 7, the mean changes in SGRQ symptoms (between-group p = 0.026), in MRC (between-group p = 0.030), and in maximal expiratory pressure (MEP; between-group p = 0.015) were better in the ghrelin group than in the placebo group. Additionally, repeated-measures analysis of variance (ANOVA) indicated significant time course effects of ghrelin versus placebo in SGRQ symptoms (p = 0.049) and MEP (p = 0.021). Ghrelin treatment was well tolerated.
Conclusions/significance: In cachectic COPD patients, with the safety profile, ghrelin administration provided improvements in symptoms and respiratory strength, despite the lack of a significant between-group difference in 6-MWD.
Trial registration: UMIN Clinical Trial Registry C000000061.
Conflict of interest statement
Figures


References
-
- Wagner PD. Possible mechanisms underlying the development of cachexia in COPD. Eur Respir J. 2008;31:492–501. - PubMed
-
- Wilson DO, Rogers RM, Wright EC, Anthonisen NR. Body weight in chronic obstructive pulmonary disease. The National Institutes of Health Intermittent Positive-Pressure Breathing Trial. Am Rev Respir Dis. 1989;139:1435–1438. - PubMed
-
- Burdet L, de Muralt B, Schutz Y, Pichard C, Fitting JW. Administration of growth hormone to underweight patients with chronic obstructive pulmonary disease. A prospective, randomized, controlled study. Am J Respir Crit Care Med. 1997;156:1800–1806. - PubMed
-
- Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest. 2007;131:4S–42S. - PubMed
-
- Zinna EM, Yarasheski KE. Exercise treatment to counteract protein wasting of chronic diseases. Curr Opin Clin Nutr Metab Care. 2003;6:87–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

