Rd9 is a naturally occurring mouse model of a common form of retinitis pigmentosa caused by mutations in RPGR-ORF15
- PMID: 22563472
- PMCID: PMC3341386
- DOI: 10.1371/journal.pone.0035865
Rd9 is a naturally occurring mouse model of a common form of retinitis pigmentosa caused by mutations in RPGR-ORF15
Abstract
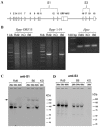
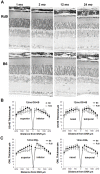
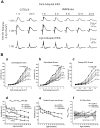
Animal models of human disease are an invaluable component of studies aimed at understanding disease pathogenesis and therapeutic possibilities. Mutations in the gene encoding retinitis pigmentosa GTPase regulator (RPGR) are the most common cause of X-linked retinitis pigmentosa (XLRP) and are estimated to cause 20% of all retinal dystrophy cases. A majority of RPGR mutations are present in ORF15, the purine-rich terminal exon of the predominant splice-variant expressed in retina. Here we describe the genetic and phenotypic characterization of the retinal degeneration 9 (Rd9) strain of mice, a naturally occurring animal model of XLRP. Rd9 mice were found to carry a 32-base-pair duplication within ORF15 that causes a shift in the reading frame that introduces a premature-stop codon. Rpgr ORF15 transcripts, but not protein, were detected in retinas from Rd9/Y male mice that exhibited retinal pathology, including pigment loss and slowly progressing decrease in outer nuclear layer thickness. The levels of rhodopsin and transducin in rod outer segments were also decreased, and M-cone opsin appeared mislocalized within cone photoreceptors. In addition, electroretinogram (ERG) a- and b-wave amplitudes of both Rd9/Y male and Rd9/Rd9 female mice showed moderate gradual reduction that continued to 24 months of age. The presence of multiple retinal features that correlate with findings in individuals with XLRP identifies Rd9 as a valuable model for use in gaining insight into ORF15-associated disease progression and pathogenesis, as well as accelerating the development and testing of therapeutic strategies for this common form of retinal dystrophy.
Conflict of interest statement
Figures



References
-
- Fishman GA, Farber MD, Derlacki DJ. X-linked retinitis pigmentosa: Profile of clinical findings. Arch Ophthalmol. 1988;106:369–375. - PubMed
-
- Shu X, Blac GC, Rice JM, Hart-Holden N, Jones A, et al. RPGR mutation analysis and disease: an update. Hum Mutat. 2007;28:322–328. - PubMed
-
- Vervoort R, Lennon A, Bird AC, Tulloch B, Axton R, et al. Mutational hot spot within a new RPGR exon in X-linked retinitis pigmentosa. Nat Genet. 2000;25:462–466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P60-DK-20572/DK/NIDDK NIH HHS/United States
- R01-EY007758/EY/NEI NIH HHS/United States
- P30-EY07003/EY/NEI NIH HHS/United States
- UL1RR024986/RR/NCRR NIH HHS/United States
- P60 DK020572/DK/NIDDK NIH HHS/United States
- R01 EY007758/EY/NEI NIH HHS/United States
- R01-EY019943/EY/NEI NIH HHS/United States
- R01 EY019943/EY/NEI NIH HHS/United States
- UL1 RR024986/RR/NCRR NIH HHS/United States
- R01 EY007961/EY/NEI NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- P30 EY007003/EY/NEI NIH HHS/United States
- R01-EY007961/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

