Functional evidence of multidrug resistance transporters (MDR) in rodent olfactory epithelium
- PMID: 22563480
- PMCID: PMC3341370
- DOI: 10.1371/journal.pone.0036167
Functional evidence of multidrug resistance transporters (MDR) in rodent olfactory epithelium
Abstract
Background: P-glycoprotein (Pgp) and multidrug resistance-associated protein (MRP1) are membrane transporter proteins which function as efflux pumps at cell membranes and are considered to exert a protective function against the entry of xenobiotics. While evidence for Pgp and MRP transporter activity is reported for olfactory tissue, their possible interaction and participation in the olfactory response has not been investigated.
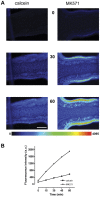
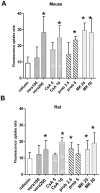
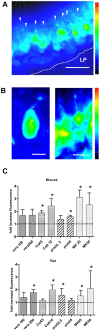
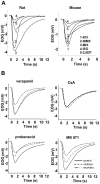
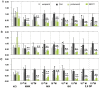
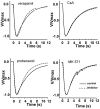
Principal findings: Functional activity of putative MDR transporters was assessed by means of the fluorometric calcein acetoxymethyl ester (calcein-AM) accumulation assay on acute rat and mouse olfactory tissue slices. Calcein-AM uptake was measured as fluorescence intensity changes in the presence of Pgp or MRP specific inhibitors. Epifluorescence microscopy measured time course analysis in the olfactory epithelium revealed significant inhibitor-dependent calcein uptake in the presence of each of the selected inhibitors. Furthermore, intracellular calcein accumulation in olfactory receptor neurons was also significantly increased in the presence of either one of the Pgp or MRP inhibitors. The presence of Pgp or MRP1 encoding genes in the olfactory mucosa of rat and mouse was confirmed by RT-PCR with appropriate pairs of species-specific primers. Both transporters were expressed in both newborn and adult olfactory mucosa of both species. To assess a possible involvement of MDR transporters in the olfactory response, we examined the electrophysiological response to odorants in the presence of the selected MDR inhibitors by recording electroolfactograms (EOG). In both animal species, MRPs inhibitors induced a marked reduction of the EOG magnitude, while Pgp inhibitors had only a minor or no measurable effect.
Conclusions: The findings suggest that both Pgp and MRP transporters are functional in the olfactory mucosa and in olfactory receptor neurons. Pgp and MRPs may be cellular constituents of olfactory receptor neurons and represent potential mechanisms for modulation of the olfactory response.
Conflict of interest statement
Figures


References
-
- Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–162. - PubMed
-
- Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. - PubMed
-
- Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204:216–237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

