The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma
- PMID: 22563484
- PMCID: PMC3341352
- DOI: 10.1371/journal.pone.0036197
The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma
Abstract
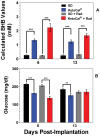
Introduction: The ketogenic diet (KD) is a high-fat, low-carbohydrate diet that alters metabolism by increasing the level of ketone bodies in the blood. KetoCal® (KC) is a nutritionally complete, commercially available 4:1 (fat:carbohydrate+protein) ketogenic formula that is an effective non-pharmacologic treatment for the management of refractory pediatric epilepsy. Diet-induced ketosis causes changes to brain homeostasis that have potential for the treatment of other neurological diseases such as malignant gliomas.
Methods: We used an intracranial bioluminescent mouse model of malignant glioma. Following implantation animals were maintained on standard diet (SD) or KC. The mice received 2×4 Gy of whole brain radiation and tumor growth was followed by in vivo imaging.
Results: Animals fed KC had elevated levels of β-hydroxybutyrate (p = 0.0173) and an increased median survival of approximately 5 days relative to animals maintained on SD. KC plus radiation treatment were more than additive, and in 9 of 11 irradiated animals maintained on KC the bioluminescent signal from the tumor cells diminished below the level of detection (p<0.0001). Animals were switched to SD 101 days after implantation and no signs of tumor recurrence were seen for over 200 days.
Conclusions: KC significantly enhances the anti-tumor effect of radiation. This suggests that cellular metabolic alterations induced through KC may be useful as an adjuvant to the current standard of care for the treatment of human malignant gliomas.
Conflict of interest statement
Figures



References
-
- Ruggiero A, Cefalo G, Garre ML, Massimino M, Colosimo C, et al. Phase II trial of temozolomide in children with recurrent high-grade glioma. J Neurooncol. 2006;77:89–94. - PubMed
-
- Nicholas MK, Lukas RV, Chmura S, Yamini B, Lesniak M, et al. Molecular heterogeneity in glioblastoma: therapeutic opportunities and challenges. Semin Oncol. 2011;38:243–253. - PubMed
-
- Niyazi M, Siefert A, Schwarz SB, Ganswindt U, Kreth FW, et al. Therapeutic options for recurrent malignant glioma. Radiother Oncol. 2011;98:1–14. - PubMed
-
- Roesler R, Brunetto AT, Abujamra AL, de Farias CB, Brunetto AL, et al. Current and emerging molecular targets in glioma. Expert Rev Anticancer Ther. 2010;10:1735–1751. - PubMed
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

