Mifepristone increases the cytotoxicity of uterine natural killer cells by acting as a glucocorticoid antagonist via ERK activation
- PMID: 22563497
- PMCID: PMC3341349
- DOI: 10.1371/journal.pone.0036413
Mifepristone increases the cytotoxicity of uterine natural killer cells by acting as a glucocorticoid antagonist via ERK activation
Abstract
Background: Mifepristone (RU486), a potent antagonist of progesterone and glucocorticoids, is involved in immune regulation. Our previous studies demonstrated that mifepristone directly augments the cytotoxicity of human uterine natural killer (uNK) cells. However, the mechanism responsible for this increase in cytotoxicity is not known. Here, we explored whether the increased cytotoxicity in uNK cells produced by mifepristone is due to either anti-progesterone or anti-glucocorticoid activity, and also investigated relevant changes in the mitogen-activated protein kinase (MAPK) pathway.
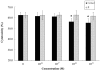
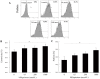
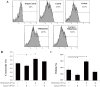
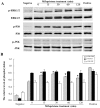
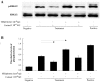
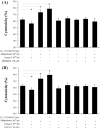
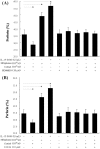
Methodology/principal findings: Uterine NK cells were isolated from decidual samples and incubated with different concentrations of progesterone, cortisol, or mifepristone. The cytotoxicity and perforin expression of uNK cells were detected by mitochondrial lactate dehydrogenase-based MTS staining and flow cytometry assays, respectively. Phosphorylation of components of the MAPK signaling pathway was detected by Western blot. Cortisol attenuated uNK cell-mediated cytotoxicity in a concentration-dependent manner whereas progesterone had no effect. Mifepristone alone increased the cytotoxicity and perforin expression of uNK cells; these effects were blocked by cortisol. Furthermore, mifepristone increased the phosphorylation of ERK1/2 in a cortisol-reversible manner. Specific ERK1/2 inhibitor PD98059 or U0126 blocked cortisol- and mifepristone-induced responses in uNK cells.
Conclusions/significance: These results suggest that mifepristone acts as a glucocorticoid antagonist to augment uNK cell-mediated cytotoxicity via ERK activation, which may be caused by increased perforin expression. These observations may reveal an important mechanism by which mifepristone upregulates the cytotoxicity of uNK cells.
Conflict of interest statement
Figures
Similar articles
-
The role of p44/42 activation in tributyltin-induced inhibition of human natural killer cells: effects of MEK inhibitors.J Appl Toxicol. 2009 Mar;29(2):165-73. doi: 10.1002/jat.1397. J Appl Toxicol. 2009. PMID: 18989867 Free PMC article.
-
Glucocorticoid receptor mediates the effect of progesterone on uterine natural killer cells.Am J Reprod Immunol. 2012 Jun;67(6):463-73. doi: 10.1111/j.1600-0897.2012.01114.x. Epub 2012 Mar 2. Am J Reprod Immunol. 2012. PMID: 22380541
-
[The role of MAPK/ERK1/2 signaling pathway in aldosterone stimulated transforming growth factor-beta1 synthesis in renal tubular epithelial cells].Zhonghua Yi Xue Za Zhi. 2006 Nov 28;86(44):3133-7. Zhonghua Yi Xue Za Zhi. 2006. PMID: 17313766 Chinese.
-
The Role of Uterine Natural Killer Cells on Recurrent Miscarriage and Recurrent Implantation Failure: From Pathophysiology to Treatment.Biomedicines. 2021 Oct 9;9(10):1425. doi: 10.3390/biomedicines9101425. Biomedicines. 2021. PMID: 34680540 Free PMC article. Review.
-
The ups and downs of structure-activity landscapes.Methods Mol Biol. 2011;672:101-17. doi: 10.1007/978-1-60761-839-3_3. Methods Mol Biol. 2011. PMID: 20838965 Free PMC article. Review.
Cited by
-
Postoperative Natural Killer Cell Dysfunction: The Prime Suspect in the Case of Metastasis Following Curative Cancer Surgery.Int J Mol Sci. 2021 Oct 21;22(21):11378. doi: 10.3390/ijms222111378. Int J Mol Sci. 2021. PMID: 34768810 Free PMC article. Review.
-
Knowledge-guided deep learning models of drug toxicity improve interpretation.Patterns (N Y). 2022 Aug 24;3(9):100565. doi: 10.1016/j.patter.2022.100565. eCollection 2022 Sep 9. Patterns (N Y). 2022. PMID: 36124309 Free PMC article.
-
Intrauterine administration of peripheral blood mononuclear cells (PBMCs) improves embryo implantation in mice by regulating local Treg/Th17 cell balance.J Reprod Dev. 2021 Dec 14;67(6):359-368. doi: 10.1262/jrd.2021-006. Epub 2021 Oct 7. J Reprod Dev. 2021. PMID: 34615838 Free PMC article.
-
Endogenous TWEAK is critical for regulating the function of mouse uterine natural killer cells in an immunological model of pregnancy loss.Immunology. 2016 May;148(1):70-82. doi: 10.1111/imm.12588. Epub 2016 Mar 2. Immunology. 2016. PMID: 27040357 Free PMC article.
-
Dexamethasone induces primary amnion epithelial cell senescence through telomere-P21 associated pathway†.Biol Reprod. 2019 Jun 1;100(6):1605-1616. doi: 10.1093/biolre/ioz048. Biol Reprod. 2019. PMID: 30927408 Free PMC article.
References
-
- Spitz IM. Progesterone receptor antagonists. Curr Opin Investig Drugs. 2006;7:882–890. - PubMed
-
- Li A, Felix JC, Minoo P, Amezcua CA, Jain JK. Effect of mifepristone on proliferation and apoptosis of Ishikawa endometrial adenocarcinoma cells. Fertil Steril. 2005;84:202–211. - PubMed
-
- Lin MF, Kawachi MH, Stallcup MR, Grunberg SM, Lin FF. Growth inhibition of androgen-insensitive human prostate carcinoma cells by a 19-norsteroid derivative agent, mifepristone. Prostate. 1995;26:194–204. - PubMed
-
- Check JH, Sansoucie L, Chern J, Dix E. Mifepristone treatment improves length and quality of survival of mice with spontaneous lung cancer. Anticancer Res. 2010;30:119–122. - PubMed
-
- Chien CH, Lai JN, Liao CF, Wang OY, Lu LM, et al. Mifepristone acts as progesterone antagonist of non-genomic responses but inhibits phytohemagglutinin-induced proliferation in human T cells. Hum Reprod. 2009;24:1968–1975. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

