R-Smad competition controls activin receptor output in Drosophila
- PMID: 22563507
- PMCID: PMC3341346
- DOI: 10.1371/journal.pone.0036548
R-Smad competition controls activin receptor output in Drosophila
Abstract
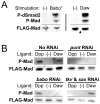
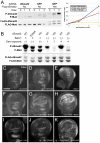
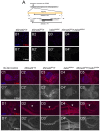
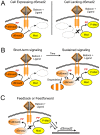
Animals use TGF-β superfamily signal transduction pathways during development and tissue maintenance. The superfamily has traditionally been divided into TGF-β/Activin and BMP branches based on relationships between ligands, receptors, and R-Smads. Several previous reports have shown that, in cell culture systems, "BMP-specific" Smads can be phosphorylated in response to TGF-β/Activin pathway activation. Using Drosophila cell culture as well as in vivo assays, we find that Baboon, the Drosophila TGF-β/Activin-specific Type I receptor, can phosphorylate Mad, the BMP-specific R-Smad, in addition to its normal substrate, dSmad2. The Baboon-Mad activation appears direct because it occurs in the absence of canonical BMP Type I receptors. Wing phenotypes generated by Baboon gain-of-function require Mad, and are partially suppressed by over-expression of dSmad2. In the larval wing disc, activated Baboon cell-autonomously causes C-terminal Mad phosphorylation, but only when endogenous dSmad2 protein is depleted. The Baboon-Mad relationship is thus controlled by dSmad2 levels. Elevated P-Mad is seen in several tissues of dSmad2 protein-null mutant larvae, and these levels are normalized in dSmad2; baboon double mutants, indicating that the cross-talk reaction and Smad competition occur with endogenous levels of signaling components in vivo. In addition, we find that high levels of Activin signaling cause substantial turnover in dSmad2 protein, providing a potential cross-pathway signal-switching mechanism. We propose that the dual activity of TGF-β/Activin receptors is an ancient feature, and we discuss several ways this activity can modulate TGF-β signaling output.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

