Evaluation of nephroprotective and immunomodulatory activities of antioxidants in combination with cisplatin against murine visceral leishmaniasis
- PMID: 22563510
- PMCID: PMC3341342
- DOI: 10.1371/journal.pntd.0001629
Evaluation of nephroprotective and immunomodulatory activities of antioxidants in combination with cisplatin against murine visceral leishmaniasis
Abstract
Background: Most available drugs against visceral leishmaniasis are toxic, and growing limitations in available chemotherapeutic strategies due to emerging resistant strains and lack of an effective vaccine against visceral leishmaniasis deepens the crisis. Antineoplastic drugs like miltefosine have in the past been effective against the parasitic infections. An antineoplastic drug, cisplatin (cis-diamminedichloroplatinum II; CDDP), is recognized as a DNA-damaging drug which also induces alteration of cell-cycle in both promastigotes and amastigotes leading to cell death. First in vivo reports from our laboratory revealed the leishmanicidal potential of cisplatin. However, high doses of cisplatin produce impairment of kidney, which can be reduced by the administration of antioxidants.
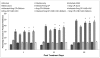
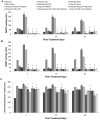
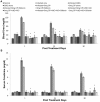
Methodology/principal findings: The present study was designed to evaluate the antileishmanial effect of cisplatin at higher doses (5 mg and 2.5 mg/kg body weight) and its combination with different antioxidants (vitamin C, vitamin E and silibinin) so as to eliminate the parasite completely and reduce the toxicity. In addition, various immunological, hematological and biochemical changes induced by it in uninfected and Leishmania donovani infected BALB/c mice were investigated.
Conclusion/significance: A significant reduction in parasite load, higher IgG2a and lower IgG1 levels, enhanced DTH responses, and greater concentration of Th1 cytokines (IFN-γ, IL-2) with a concomitant down regulation of IL-10 and IL-4 pointed towards the generation of the protective Th1 type of immune response. A combination of cisplatin with antioxidants resulted in successful reduction of nephrotoxicity by normalizing the enzymatic levels of various liver and kidney function tests. Reduction in parasite load, increase in Th1 type of immune responses, and normalization of various biochemical parameters occurred in animals treated with cisplatin in combination with various antioxidants as compared to those treated with the drug only. The above results are promising as antioxidants reduced the potential toxicity of high doses of cisplatin, making the combination a potential anti-leishmanial therapy, especially in resistant cases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- Herwaldt BL. Leishmaniasis. Lancet. 1999;354(9185):1191–1199. - PubMed
-
- Sundar S, Chatterjee M. Visceral leishmaniasis – current therapeutic modalities. Indian J Med Res. 2006;123(3):345–352. - PubMed
-
- Kshirsagar NA, Bodhe P, Kotwani RN. Targeted drug delivery in visceral leishmaniasis. J Parasit Dis. 1997;21:21–24.
-
- Thakur CP, Bhowmick S, Dolfi L, Olliaro P. Aminosidine plus sodium stibogluconate for the treatment of Indian kala-azar: a randomized dose-finding clinical trial. Trans R Soc Trop Med Hyg. 1995;89(2):219–223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

