β-Nicotinamide adenine dinucleotide attenuates lipopolysaccharide-induced inflammatory effects in a murine model of acute lung injury
- PMID: 22563684
- PMCID: PMC3678723
- DOI: 10.3109/01902148.2012.673049
β-Nicotinamide adenine dinucleotide attenuates lipopolysaccharide-induced inflammatory effects in a murine model of acute lung injury
Abstract
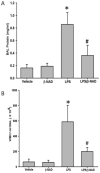
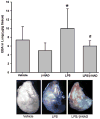
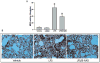
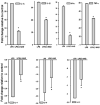
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) occur in approximately 200,000 patients per year. Studies indicate that lung endothelium plays a significant role in ALI. The authors' recent in vitro studies demonstrate a novel mechanism of β-nicotinamide adenine dinucleotide (β-NAD)-induced protection against gram-positive (pneumolysin, PLY) and gram-negative (lipopolysaccharide, LPS) toxin-induced lung endothelial cell (EC) barrier dysfunction. The objective of the current study was to evaluate the protective effect of β-NAD against LPS-induced ALI in mice. C57BL/6J mice were randomly divided into 4 groups: vehicle, β-NAD, LPS, and LPS/β-NAD. After surgery, mice were allowed to recover for 24 hours. Evans blue dye-albumin (EBA) was given through the internal jugular vein 2 hours prior to the termination of the experiments. Upon sacrificing the animals, bronchoalveolar lavage fluid (BALF) was collected and the lungs were harvested. β-NAD treatment significantly attenuated the inflammatory response by means of reducing the accumulation of cells and protein in BALF, blunting the parenchymal neutrophil infiltration, and preventing capillary leak. In addition, the histological examination demonstrated decreased interstitial edema in the LPS/β-NAD specimens, as compared to the LPS-only specimens. The mRNA levels of the anti-inflammatory cytokines were up-regulated in the LPS group treated with β-NAD compared to the LPS-only-treated group. β-NAD treatment down-regulated the mRNA levels of the proinflammatory cytokines. These findings suggest that β-NAD could be investigated as a therapeutic option against bacterial toxin-induced lung inflammation and ALI in mice.
Conflict of interest statement
Figures

References
-
- Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31(4 Suppl):S195–S199. - PubMed
-
- Razavi HM, Wang le F, Weicker S, Rohan M, Law C, McCormack DG, et al. Pulmonary neutrophil infiltration in murine sepsis: role of inducible nitric oxide synthase. Am J Respir Crit Care Med. 2004;170:227–233. - PubMed
-
- Wang le F, Patel M, Razavi HM, Weicker S, Joseph MG, McCormack DG, et al. Role of inducible nitric oxide synthase in pulmonary microvascular protein leak in murine sepsis. Am J Respir Crit Care Med. 2002;165:1634–1639. - PubMed
-
- Suda K, Tsuruta M, Eom J, Or C, Mui T, Jaw JE, et al. Acute lung injury induced cardiovascular dysfunction: effects of IL-6 and budesonide/formoterol. Am J Respir Cell Mol Biol. 2011 Jan 21;45:510–516. - PubMed
-
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
