Epigallocatechin-3-gallate induces mesothelioma cell death via H2 O2 -dependent T-type Ca2+ channel opening
- PMID: 22564432
- PMCID: PMC4118235
- DOI: 10.1111/j.1582-4934.2012.01584.x
Epigallocatechin-3-gallate induces mesothelioma cell death via H2 O2 -dependent T-type Ca2+ channel opening
Abstract
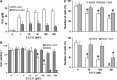
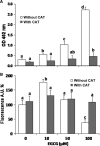
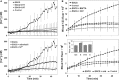
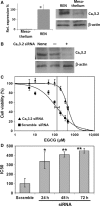
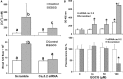
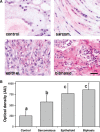
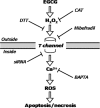
Malignant mesothelioma (MMe) is a highly aggressive, lethal tumour requiring the development of more effective therapies. The green tea polyphenol epigallocathechin-3-gallate (EGCG) inhibits the growth of many types of cancer cells. We found that EGCG is selectively cytotoxic to MMe cells with respect to normal mesothelial cells. MMe cell viability was inhibited by predominant induction of apoptosis at lower doses and necrosis at higher doses. EGCG elicited H(2) O(2) release in cell cultures, and exogenous catalase (CAT) abrogated EGCG-induced cytotoxicity, apoptosis and necrosis. Confocal imaging of fluo 3-loaded, EGCG-exposed MMe cells showed significant [Ca(2+) ](i) rise, prevented by CAT, dithiothreitol or the T-type Ca(2+) channel blockers mibefradil and NiCl(2) . Cell loading with dihydrorhodamine 123 revealed EGCG-induced ROS production, prevented by CAT, mibefradil or the Ca(2+) chelator BAPTA-AM. Direct exposure of cells to H(2) O(2) produced similar effects on Ca(2+) and ROS, and these effects were prevented by the same inhibitors. Sensitivity of REN cells to EGCG was correlated with higher expression of Ca(v) 3.2 T-type Ca(2+) channels in these cells, compared to normal mesothelium. Also, Ca(v) 3.2 siRNA on MMe cells reduced in vitro EGCG cytotoxicity and abated apoptosis and necrosis. Intriguingly, Ca(v) 3.2 expression was observed in malignant pleural mesothelioma biopsies from patients, but not in normal pleura. In conclusion, data showed the expression of T-type Ca(2+) channels in MMe tissue and their role in EGCG selective cytotoxicity to MMe cells, suggesting the possible use of these channels as a novel MMe pharmacological target.
© 2012 The Authors Journal of Cellular and Molecular Medicine © 2012 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures

References
-
- Pass HI, Vogelzang N, Hahn S, et al. Malignant pleural mesothelioma. Curr Probl Cancer. 2004;28:93–174. - PubMed
-
- Belli C, Fennell D, Giovannini M, et al. Malignant pleural mesothelioma: current treatments and emerging drugs. Expert Opin Emerg Drugs. 2009;14:423–37. - PubMed
-
- Sueoka E, Sueoka N, Okabe S, et al. Expression of the tumour necrosis factor alpha gene and early response genes by nodularin, a liver tumour promoter, in primary cultured rat hepatocytes. J Cancer Res Clin Oncol. 1997;123:413–9. - PubMed
-
- Fennell DA, Gaudino G, O'Byrne KJ, et al. Advances in the systemic therapy of malignant pleural mesothelioma. Nat Clin Pract Oncol. 2008;5:136–47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

