Somatic GNAS mutation causes widespread and diffuse pituitary disease in acromegalic patients with McCune-Albright syndrome
- PMID: 22564667
- PMCID: PMC3791436
- DOI: 10.1210/jc.2012-1274
Somatic GNAS mutation causes widespread and diffuse pituitary disease in acromegalic patients with McCune-Albright syndrome
Abstract
Context: McCune-Albright syndrome (MAS) is caused by sporadic mutations of the GNAS. Patients exhibit features of acromegaly. In most patients, GH-secreting pituitary adenomas have been held responsible for this presentation. However, surgical adenomectomy rarely eliminates excess GH production.
Objective: The aim of this study was to elucidate pituitary pathology in patients with MAS and to explain the basis of failure of adenomectomy to eliminate GH hypersecretion.
Design and setting: We conducted a case series at the National Institutes of Health.
Intervention(s): Interventions included medical therapy and transsphenoidal surgery.
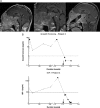
Patients and main outcome measures: We studied clinical and imaging features and the histology and molecular features of the pituitary of four acromegalic MAS patients.
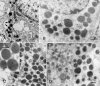
Results: We identified widespread and diffuse pituitary gland disease. The primary pathological changes were characterized by hyperplastic and neoplastic change, associated with overrepresentation of somatotroph cells in structurally intact tissue areas. Genetic analysis of multiple microdissected samples of any type of histological area consistently revealed identical GNAS mutations in individual patients. The only patient with remission after surgery received complete hypophysectomy in addition to removal of multiple GH-secreting tumors.
Conclusions: These findings indicate developmental effects of GNAS mutation on the entire anterior pituitary gland. The pituitary of individual cases contains a spectrum of changes with regions of normal appearing gland, hyperplasia, and areas of fully developed adenoma formation, as well as transitional stages between these entities. The primary change underlying acromegaly in MAS patients is somatotroph hyperplasia involving the entire pituitary gland, with or without development of somatotroph adenoma. Thus, successful clinical management, whether it is medical, surgical, or via irradiation, must target the entire pituitary, not just the adenomas evident on imaging.
Figures



References
-
- de Sanctis C, Lala R, Matarazzo P, Balsamo A, Bergamaschi R, Cappa M, Cisternino M, de Sanctis V, Lucci M, Franzese A, Ghizzoni L, Pasquino AM, Segni M, Rigon F, Saggese G, Bertelloni S, Buzi F. 1999. McCune-Albright syndrome: a longitudinal clinical study of 32 patients. J Pediatr Endocrinol Metab 12:817–826 - PubMed
-
- Cuttler L, Jackson JA, Saeed uz-Zafar M, Levitsky LL, Mellinger RC, Frohman LA. 1989. Hypersecretion of growth hormone and prolactin in McCune-Albright syndrome. J Clin Endocrinol Metab 68:1148–1154 - PubMed
-
- Pun KK, Chan G, Kung A, Lam K, Chan FL, Wang C. 1989. McCune-Albright syndrome with acromegaly. Horm Metab Res 21:527–528 - PubMed
-
- Chanson P, Dib A, Visot A, Derome PJ. 1994. McCune-Albright syndrome and acromegaly: clinical studies and responses to treatment in five cases. Eur J Endocrinol 131:229–234 - PubMed
-
- Bhansali A, Sharma BS, Sreenivasulu P, Singh P, Vashisth RK, Dash RJ. 2003. Acromegaly with fibrous dysplasia: McCune-Albright Syndrome—clinical studies in 3 cases and brief review of literature. Endocr J 50:793–799 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

