O-GlcNAcylation of kinases
- PMID: 22564745
- PMCID: PMC3387735
- DOI: 10.1016/j.bbrc.2012.04.124
O-GlcNAcylation of kinases
Abstract
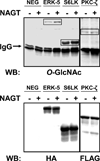
Recent evidence indicates that site-specific crosstalk between O-GlcNAcylation and phosphorylation and the O-GlcNAcylation of kinases play an important role in regulating cell signaling. However, relatively few kinases have been analyzed for O-GlcNAcylation. Here, we identify additional kinases that are substrates for O-GlcNAcylation using an in vitro OGT assay on a functional kinase array. Forty-two kinases were O-GlcNAcylated in vitro, representing 39% of the kinases on the array. In addition, we confirmed the in vivo O-GlcNAcylation of three identified kinases. Our results suggest that O-GlcNAcylation may directly regulate a substantial number of kinases and illustrates the increasingly complex relationship between O-GlcNAcylation and phosphorylation in cellular signaling.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


References
-
- Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. - PubMed
-
- Yang WH, Kim JE, Nam HW, Ju JW, Kim HS, Kim YS, Cho JW. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol. 2006;8:1074–1083. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

