Calcium-dependent physiologic and pathologic stimulus-metabolic response coupling in hepatocytes
- PMID: 22564906
- PMCID: PMC3391328
- DOI: 10.1016/j.ceca.2012.04.009
Calcium-dependent physiologic and pathologic stimulus-metabolic response coupling in hepatocytes
Abstract
A recurrent paradigm in calcium signaling is the coordination of the target response of the calcium signal with activation of metabolic energy production to support that response. This occurs in many tissues, including cardiac and skeletal muscle where contractile activity and ATP production are coordinately regulated by the frequency and amplitude of calcium transients, endocrine and exocrine cells that use calcium to drive the secretory process, and hepatocytes where the downstream targets of calcium include both catabolic and anabolic processes. The primary mechanism by which calcium enhances the capacity for energy production is through calcium-dependent stimulation of mitochondrial oxidative metabolism, achieved by increasing NADH production and respiratory chain flux. Although this enhances energy supply, it also has the potential for deleterious consequences resulting from increased generation of reactive oxygen species (ROS). The negative consequences of calcium-dependent mitochondrial activation can be ameliorated when the underlying cytosolic calcium signals occur as brief calcium spikes or oscillations, with signal strength encoded through the spike frequency (frequency modulation). Frequency modulation increases signal fidelity, and reduces pathological effects of calcium, including excess mitochondrial ROS production and apoptotic or necrotic outcomes. The present article reviews these issues using data obtained in hepatocytes under physiologic and pathologic conditions.
Published by Elsevier India Pvt Ltd.
Figures
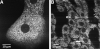
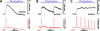
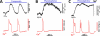
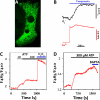



References
-
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. - PubMed
-
- Clapham DE. Calcium signaling. Cell. 1995;80:259–268. - PubMed
-
- Gaspers LD, Thomas AP. Calcium signaling in liver. Cell Calcium. 2005;38:329–342. - PubMed
-
- Gaspers LD, Pierobon N, Thomas AP. Calcium signaling. In: Dufour JF, Clavien P-A, editors. Signaling Pathways in Liver Disease. Springer-Verlag GmbH & Co. KG; Heidelberg, Germany: 2005. pp. 211–221.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

