Prenatal synthetic glucocorticoid treatment changes DNA methylation states in male organ systems: multigenerational effects
- PMID: 22564977
- PMCID: PMC3422463
- DOI: 10.1210/en.2011-2160
Prenatal synthetic glucocorticoid treatment changes DNA methylation states in male organ systems: multigenerational effects
Abstract
Prenatal synthetic glucocorticoids (sGC) are administered to pregnant women at risk of delivering preterm, approximately 10% of all pregnancies. Animal studies have demonstrated that offspring exposed to elevated glucocorticoids, either by administration of sGC or as a result of maternal stress, are at increased risk of developing behavioral, endocrine, and metabolic abnormalities. DNA methylation is a covalent modification of DNA that plays a critical role in long-lasting programming of gene expression. Here we tested the hypothesis that prenatal sGC treatment has both acute and long-term effects on DNA methylation states in the fetus and offspring and that these effects extend into a subsequent generation. Pregnant guinea pigs were treated with sGC in late gestation, and methylation analysis by luminometric methylation assay was undertaken in organs from fetuses and offspring across two generations. Expression of genes that modify the epigenetic state were measured by quantitative real-time PCR. Results indicate that there are organ-specific developmental trajectories of methylation in the fetus and newborn. Furthermore, these trajectories are substantially modified by intrauterine exposure to sGC. These sGC-induced changes in DNA methylation remain into adulthood and are evident in the next generation. Furthermore, prenatal sGC exposure alters the expression of several genes encoding proteins that modulate the epigenetic state. Several of these changes are long lasting and are also present in the next generation. These data support the hypothesis that prenatal sGC exposure leads to broad changes in critical components of the epigenetic machinery and that these effects can pass to the next generation.
Figures


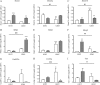
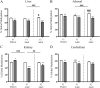




References
-
- Challis JRG, Matthews SG, Gibb W, Lye SJ. 2000. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev 21:514–550 - PubMed
-
- 2001. Antenatal corticosteroids revisited: repeat courses—National Institutes of Health Consensus Development Conference Statement, August 17–18, 2000. Obstet Gynecol 98:144–150 - PubMed
-
- Liggins GC, Howie RN. 1972. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 50:515–525 - PubMed
-
- Brocklehurst P, Gates S, McKenzie-McHarg K, Alfirevic Z, Chamberlain G. 1999. Are we prescribing multiple courses of antenatal corticosteroids? A survey of practice in the U.K. Br J Obstet Gynaecol 106:977–979 - PubMed
-
- Quinlivan JA, Evans SF, Dunlop SA, Beazley LD, Newnham JP. 1998. Use of corticosteroids by Australian obstetricians—a survey of clinical practice. Aust N Z J Obstet Gynaecol 38:1–7 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

