IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity
- PMID: 22565311
- PMCID: PMC3366399
- DOI: 10.1172/JCI59535
IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity
Abstract
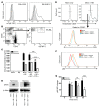
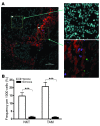
Vα24-invariant NKT cells inhibit tumor growth by targeting tumor-associated macrophages (TAMs). Tumor progression therefore requires that TAMs evade NKT cell activity through yet-unknown mechanisms. Here we report that a subset of cells in neuroblastoma (NB) cell lines and primary tumors expresses membrane-bound TNF-α (mbTNF-α). These proinflammatory tumor cells induced production of the chemokine CCL20 from TAMs via activation of the NF-κB signaling pathway, an effect that was amplified in hypoxia. Flow cytometry analyses of human primary NB tumors revealed selective accumulation of CCL20 in TAMs. Neutralization of the chemokine inhibited in vitro migration of NKT cells toward tumor-conditioned hypoxic monocytes and localization of NKT cells to NB grafts in mice. We also found that hypoxia impaired NKT cell viability and function. Thus, CCL20-producing TAMs served as a hypoxic trap for tumor-infiltrating NKT cells. IL-15 protected antigen-activated NKT cells from hypoxia, and transgenic expression of IL-15 in adoptively transferred NKT cells dramatically enhanced their antimetastatic activity in mice. Thus, tumor-induced chemokine production in hypoxic TAMs and consequent chemoattraction and inhibition of NKT cells represents a mechanism of immune escape that can be reversed by adoptive immunotherapy with IL-15-transduced NKT cells.
Figures





References
-
- Swann J, Crowe NY, Hayakawa Y, Godfrey DI, Smyth MJ. Regulation of antitumour immunity by CD1d-restricted NKT cells. Immunol Cell Biol. 2004;82(3):323–331. - PubMed
-
- Swann JB, Coquet JM, Smyth MJ, Godfrey DI. CD1-restricted T cells and tumor immunity. Curr Top Microbiol Immunol. 2007;314:293–323. - PubMed
-
- Yanagisawa K, Seino K, Ishikawa Y, Nozue M, Todoroki T, Fukao K. Impaired proliferative response of V alpha 24 NKT cells from cancer patients against alpha-galactosylceramide. J Immunol. 2002;168(12):6494–6499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

