TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice
- PMID: 22565312
- PMCID: PMC3366391
- DOI: 10.1172/JCI45414
TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice
Abstract
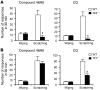
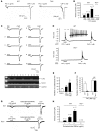
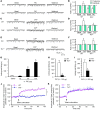
Itch, also known as pruritus, is a common, intractable symptom of several skin diseases, such as atopic dermatitis and xerosis. TLRs mediate innate immunity and regulate neuropathic pain, but their roles in pruritus are elusive. Here, we report that scratching behaviors induced by histamine-dependent and -independent pruritogens are markedly reduced in mice lacking the Tlr3 gene. TLR3 is expressed mainly by small-sized primary sensory neurons in dorsal root ganglions (DRGs) that coexpress the itch signaling pathway components transient receptor potential subtype V1 and gastrin-releasing peptide. Notably, we found that treatment with a TLR3 agonist induces inward currents and action potentials in DRG neurons and elicited scratching in WT mice but not Tlr3(-/-) mice. Furthermore, excitatory synaptic transmission in spinal cord slices and long-term potentiation in the intact spinal cord were impaired in Tlr3(-/-) mice but not Tlr7(-/-) mice. Consequently, central sensitization-driven pain hypersensitivity, but not acute pain, was impaired in Tlr3(-/-) mice. In addition, TLR3 knockdown in DRGs also attenuated pruritus in WT mice. Finally, chronic itch in a dry skin condition was substantially reduced in Tlr3(-/-) mice. Our findings demonstrate a critical role of TLR3 in regulating sensory neuronal excitability, spinal cord synaptic transmission, and central sensitization. TLR3 may serve as a new target for developing anti-itch treatment.
Figures
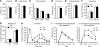




References
-
- Town T, Jeng D, Alexopoulou L, Tan J, Flavell RA. Microglia recognize double-stranded RNA via TLR3. J Immunol. 2006;176(6):3804–3812. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

