Age-related aneuploidy through cohesion exhaustion
- PMID: 22565322
- PMCID: PMC3367239
- DOI: 10.1038/embor.2012.54
Age-related aneuploidy through cohesion exhaustion
Abstract
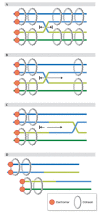
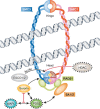
The trend of women to become pregnant when older than in previous generations poses a paramount medical problem, for oocytes are particularly prone to chromosome missegregation, and aneuploidy increases with age. Recent data strongly suggest that as oocyte age increases sister chromatid cohesion is weakened or lost. Cohesin deterioration seems to contribute significantly to age-dependent aneuploidy, as discussed in this review.
Conflict of interest statement
The author declares that he has no conflict of interest.
Figures

References
-
- Hassold T, Hunt P (2001) To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2: 280–291 - PubMed
-
- Hassold T, Hall H, Hunt P (2007) The origin of human aneuploidy: where we have been, where we are going. Hum Mol Genet 16: R203–R208 - PubMed
-
- Homer H (2007) Ageing, aneuploidy and meiosis: eggs in a race against time. Yearbook of Obstetrics and Gynaecology 12: 139–158
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

