RAIN-Droplet: a novel 3D in vitro angiogenesis model
- PMID: 22565576
- PMCID: PMC4043634
- DOI: 10.1038/labinvest.2012.77
RAIN-Droplet: a novel 3D in vitro angiogenesis model
Abstract
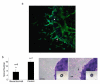
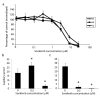
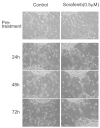
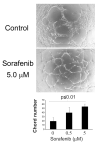
Angiogenesis is fundamentally required for the initialization, development and metastatic spread of cancer. A rapidly expanding number of new experimental, chemical modulators of endothelial cell function have been described for the therapeutic inhibition of angiogenesis in cancer. Despite this expansion, there has been very limited parallel growth of in vitro angiogenesis models or experimental tools. Here we present the Responsive Angiogenic Implanted Network (RAIN)-Droplet model and novel angiogenesis assay using an endothelial cell culture model of microvascular endothelial cells encapsulated in a spontaneously self-assembling, toroidal hydrogel droplet uniquely yielding discrete, pre-formed, angiogenic networks that may be embedded in 3D matrices. On embedding, radial growth of capillary-like sprouts and cell invasion was observed. The sprouts formed not only as outgrowths from endothelial cells on the surface of the droplets, but also, uniquely, from the pre-formed network structures within the droplet. We demonstrate proof of principle for the utility of the model showing significant inhibition of sprout formation (P<0.001) in the presence of bevacizumab, an anti-angiogenic antibody. Using the RAIN-Droplet assay, we also demonstrate a novel dose-dependent pro-angiogenic function for the characteristically anti-angiogenic multi-kinase inhibitor sorafenib. Exposure of endothelial cells in 3D culture to low, non-lethal doses (<1 μM) of sorafenib after initiation of sprouting resulted in the formation of significantly (P<0.05) more endothelial sprouts compared with controls over a 48-h period. Higher doses of sorafenib (5 μM) resulted in a significant (P<0.05) reduction of sprouting over the same time period. The RAIN-Droplet model is a highly versatile and simply constructed 3D focal sprouting approach well suited for the study of vascular morphogenesis and for preclinical testing of drugs. Furthermore, the RAIN-Droplet model has facilitated the discovery of a novel pro-angiogenic capacity for sorafenib, which may impact the clinical application and dosing regimen of that drug.
Figures



Similar articles
-
Sorafenib enhances the in vitro anti-endothelial effects of low dose (metronomic) chemotherapy.Oncol Rep. 2010 Oct;24(4):1049-58. doi: 10.3892/or.2010.1049. Oncol Rep. 2010. PMID: 20811688
-
Targeting angiogenesis in bladder cancer.Curr Oncol Rep. 2009 May;11(3):244-9. doi: 10.1007/s11912-009-0034-2. Curr Oncol Rep. 2009. PMID: 19336017 Review.
-
A Vascular Endothelial Growth Factor-Dependent Sprouting Angiogenesis Assay Based on an In Vitro Human Blood Vessel Model for the Study of Anti-Angiogenic Drugs.EBioMedicine. 2018 Jan;27:225-236. doi: 10.1016/j.ebiom.2017.12.014. Epub 2017 Dec 20. EBioMedicine. 2018. PMID: 29289530 Free PMC article.
-
Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity.J Clin Oncol. 2008 Aug 1;26(22):3709-14. doi: 10.1200/JCO.2007.10.8332. J Clin Oncol. 2008. PMID: 18669456 Free PMC article. Clinical Trial.
-
Update on angiogenesis inhibitors.Curr Opin Oncol. 2005 Nov;17(6):578-83. doi: 10.1097/01.cco.0000183672.15133.ab. Curr Opin Oncol. 2005. PMID: 16224236 Review.
Cited by
-
Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine.Stem Cells Int. 2018 Jul 30;2018:2495848. doi: 10.1155/2018/2495848. eCollection 2018. Stem Cells Int. 2018. PMID: 30154861 Free PMC article. Review.
-
The interplay of cells, polymers, and vascularization in three-dimensional lung models and their applications in COVID-19 research and therapy.Stem Cell Res Ther. 2023 Apr 28;14(1):114. doi: 10.1186/s13287-023-03341-4. Stem Cell Res Ther. 2023. PMID: 37118810 Free PMC article. Review.
-
Emerging Technologies for Cancer Research: Towards Personalized Medicine with Microfluidic Platforms and 3D Tumor Models.Curr Med Chem. 2018;25(35):4616-4637. doi: 10.2174/0929867325666180605122633. Curr Med Chem. 2018. PMID: 29874987 Free PMC article. Review.
-
In vitro 3D angiogenesis assay in egg white matrix: comparison to Matrigel, compatibility to various species, and suitability for drug testing.Lab Invest. 2014 Mar;94(3):340-9. doi: 10.1038/labinvest.2013.150. Epub 2014 Jan 6. Lab Invest. 2014. PMID: 24395110
-
Endothelial cells expressing low levels of CD143 (ACE) exhibit enhanced sprouting and potency in relieving tissue ischemia.Angiogenesis. 2014 Jul;17(3):617-30. doi: 10.1007/s10456-014-9414-9. Epub 2014 Jan 11. Angiogenesis. 2014. PMID: 24414940 Free PMC article.
References
-
- Nagy JA, Dvorak AM, Dvorak HF. VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol. 2007;2:251–75. - PubMed
-
- Taraboletti G, Giavazzi R. Modelling approaches for angiogenesis. Eur J Cancer. 2004;40:881–9. - PubMed
-
- Folkman J. Angiogenesis: An organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 CA097248/CA/NCI NIH HHS/United States
- R01-DE15948/DE/NIDCR NIH HHS/United States
- P50-CA97248/CA/NCI NIH HHS/United States
- R01 DE014601/DE/NIDCR NIH HHS/United States
- R01 DE021139/DE/NIDCR NIH HHS/United States
- R01-DE14601/DE/NIDCR NIH HHS/United States
- R01 DE016586/DE/NIDCR NIH HHS/United States
- R01-DE16586/DE/NIDCR NIH HHS/United States
- R01 DE015948/DE/NIDCR NIH HHS/United States
- R21-DE19279/DE/NIDCR NIH HHS/United States
- R01-DE021139/DE/NIDCR NIH HHS/United States
- R21 DE019279/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources

