Zebrafish homologs of genes within 16p11.2, a genomic region associated with brain disorders, are active during brain development, and include two deletion dosage sensor genes
- PMID: 22566537
- PMCID: PMC3484866
- DOI: 10.1242/dmm.009944
Zebrafish homologs of genes within 16p11.2, a genomic region associated with brain disorders, are active during brain development, and include two deletion dosage sensor genes
Abstract
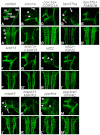
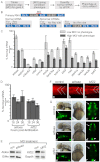
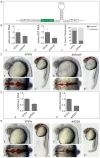
Deletion or duplication of one copy of the human 16p11.2 interval is tightly associated with impaired brain function, including autism spectrum disorders (ASDs), intellectual disability disorder (IDD) and other phenotypes, indicating the importance of gene dosage in this copy number variant region (CNV). The core of this CNV includes 25 genes; however, the number of genes that contribute to these phenotypes is not known. Furthermore, genes whose functional levels change with deletion or duplication (termed 'dosage sensors'), which can associate the CNV with pathologies, have not been identified in this region. Using the zebrafish as a tool, a set of 16p11.2 homologs was identified, primarily on chromosomes 3 and 12. Use of 11 phenotypic assays, spanning the first 5 days of development, demonstrated that this set of genes is highly active, such that 21 out of the 22 homologs tested showed loss-of-function phenotypes. Most genes in this region were required for nervous system development - impacting brain morphology, eye development, axonal density or organization, and motor response. In general, human genes were able to substitute for the fish homolog, demonstrating orthology and suggesting conserved molecular pathways. In a screen for 16p11.2 genes whose function is sensitive to hemizygosity, the aldolase a (aldoaa) and kinesin family member 22 (kif22) genes were identified as giving clear phenotypes when RNA levels were reduced by ∼50%, suggesting that these genes are deletion dosage sensors. This study leads to two major findings. The first is that the 16p11.2 region comprises a highly active set of genes, which could present a large genetic target and might explain why multiple brain function, and other, phenotypes are associated with this interval. The second major finding is that there are (at least) two genes with deletion dosage sensor properties among the 16p11.2 set, and these could link this CNV to brain disorders such as ASD and IDD.
Figures




References
-
- Almgren M., Schalling M., Lavebratt C. (2008). Idiopathic megalencephaly-possible cause and treatment opportunities: from patient to lab. Eur. J. Paediatr. Neurol. 12, 438–445 - PubMed
-
- Amaral D. G., Schumann C. M., Nordahl C. W. (2008). Neuroanatomy of autism. Trends Neurosci. 31, 137–145 - PubMed
-
- An M., Luo R., Henion P. D. (2002). Differentiation and maturation of zebrafish dorsal root and sympathetic ganglion neurons. J. Comp. Neurol. 446, 267–275 - PubMed
-
- Bardakjian T. M., Kwok S., Slavotinek A. M., Schneider A. S. (2010). Clinical report of microphthalmia and optic nerve coloboma associated with a de novo microdeletion of chromosome 16p11.2. Am. J. Med. Genet. A 152A, 3120–3123 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

