Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia
- PMID: 22566601
- PMCID: PMC3383014
- DOI: 10.1182/blood-2011-11-393975
Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia
Abstract
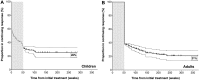
Treatments for immune thrombocytopenic purpura (ITP) providing durable platelet responses without continued dosing are limited. Whereas complete responses (CRs) to B-cell depletion in ITP usually last for 1 year in adults, partial responses (PRs) are less durable. Comparable data do not exist for children and 5-year outcomes are unavailable. Patients with ITP treated with rituximab who achieved CRs and PRs (platelets > 150 × 10(9)/L or 50-150 × 10(9)/L, respectively) were selected to be assessed for duration of their response; 72 adults whose response lasted at least 1 year and 66 children with response of any duration were included. Patients had baseline platelet counts < 30 × 10(9)/L; 95% had ITP of > 6 months in duration. Adults and children each had initial overall response rates of 57% and similar 5-year estimates of persisting response (21% and 26%, respectively). Children did not relapse after 2 years from initial treatment whereas adults did. Initial CR and prolonged B-cell depletion predicted sustained responses whereas prior splenectomy, age, sex, and duration of ITP did not. No novel or substantial long-term clinical toxicity was observed. In summary, 21% to 26% of adults and children with chronic ITP treated with standard-dose rituximab maintained a treatment-free response for at least 5 years without major toxicity. These results can inform clinical decision-making.
Figures


References
-
- Valentine MA, Meier KE, Rossie S, Clark EA. Phosphorylation of the CD20 phosphoprotein in resting B lymphocytes. Regulation by protein kinase C. J Biol Chem. 1989;264(19):11282–11287. - PubMed
-
- Reff ME, Carner K, Chambers KS, et al. Depletion of B-cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83(2):435–445. - PubMed
-
- Braendstrup P, Bjerrum OW, Nielsen OJ, et al. Rituximab chimeric anti-CD20 monoclonal antibody treatment for adult refractory idiopathic thrombocytopenic purpura. Am J Hematol. 2005;78(4):275–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

